Synthesis of rare-earth metal compounds through enhanced reactivity of alkali halides at high pressures
- PMID: 36697723
- PMCID: PMC9814685
- DOI: 10.1038/s42004-022-00736-x
Synthesis of rare-earth metal compounds through enhanced reactivity of alkali halides at high pressures
Abstract
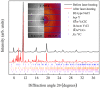
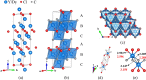
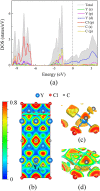
Chemical stability of the alkali halides NaCl and KCl has allowed for their use as inert media in high-pressure high-temperature experiments. Here we demonstrate the unexpected reactivity of the halides with metals (Y, Dy, and Re) and iron oxide (FeO) in a laser-heated diamond anvil cell, thus providing a synthetic route for halogen-containing binary and ternary compounds. So far unknown chlorides, Y2Cl and DyCl, and chloride carbides, Y2ClC and Dy2ClC, were synthesized at ~40 GPa and 2000 K and their structures were solved and refined using in situ single-crystal synchrotron X-ray diffraction. Also, FeCl2 with the HP-PdF2-type structure, previously reported at 108 GPa, was synthesized at ~160 GPa and 2100 K. The results of our ab initio calculations fully support experimental findings and reveal the electronic structure and chemical bonding in these compounds.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
High-pressure synthesis of dysprosium carbides.Front Chem. 2023 Jun 13;11:1210081. doi: 10.3389/fchem.2023.1210081. eCollection 2023. Front Chem. 2023. PMID: 37383952 Free PMC article.
-
High-Pressure Yttrium Borate oC20-YBO3 and Yttrium Orthocarbonate hR39-Y3(CO4)2 Synthesized at Megabar Pressures.Inorg Chem. 2025 Mar 17;64(10):5098-5104. doi: 10.1021/acs.inorgchem.4c05308. Epub 2025 Mar 2. Inorg Chem. 2025. PMID: 40025706
-
Unraveling the Bonding Complexity of Polyhalogen Anions: High-Pressure Synthesis of Unpredicted Sodium Chlorides Na2Cl3 and Na4Cl5 and Bromide Na4Br5.JACS Au. 2023 Jun 5;3(6):1634-1641. doi: 10.1021/jacsau.3c00090. eCollection 2023 Jun 26. JACS Au. 2023. PMID: 37388691 Free PMC article.
-
Novel High-Pressure Yttrium Carbide γ-Y_{4}C_{5} Containing [C_{2}] and Nonlinear [C_{3}] Units with Unusually Large Formal Charges.Phys Rev Lett. 2021 Sep 24;127(13):135501. doi: 10.1103/PhysRevLett.127.135501. Phys Rev Lett. 2021. PMID: 34623860
-
Synthesis of Binary Transition Metal Nitrides, Carbides and Borides from the Elements in the Laser-Heated Diamond Anvil Cell and Their Structure-Property Relations.Materials (Basel). 2011 Sep 28;4(10):1648-1692. doi: 10.3390/ma4101648. Materials (Basel). 2011. PMID: 28824101 Free PMC article. Review.
Cited by
-
Synthesis of LaCN3, TbCN3, CeCN5, and TbCN5 Polycarbonitrides at Megabar Pressures.J Am Chem Soc. 2024 Jul 3;146(26):18161-18171. doi: 10.1021/jacs.4c06068. Epub 2024 Jun 25. J Am Chem Soc. 2024. PMID: 38916483 Free PMC article.
-
High-pressure chemistry.Commun Chem. 2025 Jun 2;8(1):168. doi: 10.1038/s42004-025-01559-2. Commun Chem. 2025. PMID: 40456905 Free PMC article.
-
Stabilization Of The CN3 5- Anion In Recoverable High-pressure Ln3 O2 (CN3 ) (Ln=La, Eu, Gd, Tb, Ho, Yb) Oxoguanidinates.Angew Chem Int Ed Engl. 2023 Nov 20;62(47):e202311516. doi: 10.1002/anie.202311516. Epub 2023 Oct 16. Angew Chem Int Ed Engl. 2023. PMID: 37768278 Free PMC article.
References
-
- Dorogokupets PI, Dewaele A. Equations of state of MgO, au, pt, NaCl-B1, and NaCl-B2: Internally consistent high-temperature pressure scales. High. Press. Res. 2007;27:431–446. doi: 10.1080/08957950701659700. - DOI
-
- Decker DL. Equation of state of NaCl and its use as a pressure gauge in high‐pressure research. J. Appl. Phys. 1965;36:157–161. doi: 10.1063/1.1713864. - DOI
-
- Hu JZ, et al. X-ray diffraction and laser heating: application of a moissanite anvil cell. J. Phys. -Condens. Matter. 2002;14:10479–10481. doi: 10.1088/0953-8984/14/44/318. - DOI
-
- Armentrout M, Kavner A. High pressure, high temperature equation of state for Fe2SiO4ringwoodite and implications for the Earth’s transition zone. Geophys. Res. Lett. 2011;38:n/a–n/a. doi: 10.1029/2011GL046949. - DOI
-
- Langerome B, et al. Probing NaCl at high pressure through optical studies and Ab initio calculations. J. Phys. Chem. C. 2019;123:15724–15728. doi: 10.1021/acs.jpcc.9b02915. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

