How neocarcerand Octacid4 self-assembles with guests into irreversible noncovalent complexes and what accelerates the assembly
- PMID: 36697791
- PMCID: PMC9814096
- DOI: 10.1038/s42004-022-00624-4
How neocarcerand Octacid4 self-assembles with guests into irreversible noncovalent complexes and what accelerates the assembly
Abstract
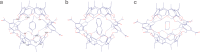
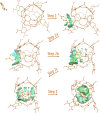
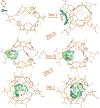
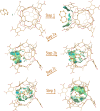
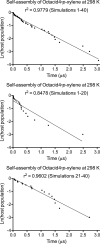
Cram's supramolecular capsule Octacid4 can irreversibly and noncovalently self-assemble with small-molecule guests at room temperature, but how they self-assemble and what accelerates their assembly remain poorly understood. This article reports 81 distinct Octacid4•guest self-assembly pathways captured in unrestricted, unbiased molecular dynamics simulations. These pathways reveal that the self-assembly was initiated by the guest interaction with the cavity portal exterior of Octacid4 to increase the portal collisions that led to the portal expansion for guest ingress, and completed by the portal contraction caused by the guest docking inside the cavity to impede guest egress. The pathways also reveal that the self-assembly was accelerated by engaging populated host and guest conformations for the exterior interaction to increase the portal collision frequency. These revelations may help explain why the presence of an exterior binding site at the rim of the enzyme active site is a fundamental feature of fast enzymes such as acetylcholinesterase and why small molecules adopt local minimum conformations when binding to proteins. Further, these revelations suggest that irreversible noncovalent complexes with fast assembly rates could be developed-by engaging populated host and guest conformations for the exterior interactions-for materials technology, data storage and processing, molecular sensing and tagging, and drug therapy.
© 2022. The Author(s).
Conflict of interest statement
The author declares no competing interests.
Figures
Similar articles
-
How the water-soluble hemicarcerand incarcerates guests at room temperature decoded with modular simulations.Commun Chem. 2021 Mar 1;4(1):26. doi: 10.1038/s42004-021-00469-3. Commun Chem. 2021. PMID: 36697600 Free PMC article.
-
Different and Often Opposing Forces Drive the Encapsulation and Multiple Exterior Binding of Charged Guests to a M4 L6 Supramolecular Vessel in Water.Chemistry. 2017 Nov 27;23(66):16813-16818. doi: 10.1002/chem.201703202. Epub 2017 Nov 8. Chemistry. 2017. PMID: 28898458
-
Equilibrium isotope effects on noncovalent interactions in a supramolecular host-guest system.J Am Chem Soc. 2012 Feb 1;134(4):2057-66. doi: 10.1021/ja2067324. Epub 2012 Jan 24. J Am Chem Soc. 2012. PMID: 22145944
-
Noncovalent tailoring of coordination complexes by resorcin[4]arene-based supramolecular hosts.Dalton Trans. 2023 May 22;52(20):6604-6618. doi: 10.1039/d3dt00710c. Dalton Trans. 2023. PMID: 37128873 Review.
-
Molecular Recognition in the Colloidal World.Acc Chem Res. 2017 Nov 21;50(11):2756-2766. doi: 10.1021/acs.accounts.7b00370. Epub 2017 Oct 6. Acc Chem Res. 2017. PMID: 28984441 Review.
References
-
- Pang Y-P. Insect acetylcholinesterase as a target for effective and environmentally safe insecticides. Adv. Insect Physiol. 2014;46:435–494. doi: 10.1016/B978-0-12-417010-0.00006-9. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

