Actinomycetes-derived imine reductases with a preference towards bulky amine substrates
- PMID: 36697820
- PMCID: PMC9814587
- DOI: 10.1038/s42004-022-00743-y
Actinomycetes-derived imine reductases with a preference towards bulky amine substrates
Abstract
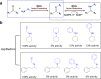
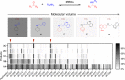
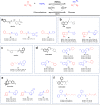
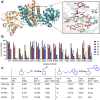
Since imine reductases (IREDs) were reported to catalyze the reductive amination reactions, they became particularly attractive for producing chiral amines. Though diverse ketones and aldehydes have been proved to be excellent substrates of IREDs, bulky amines have been rarely transformed. Here we report the usage of an Increasing-Molecule-Volume-Screening to identify a group of IREDs (IR-G02, 21, and 35) competent for accepting bulky amine substrates. IR-G02 shows an excellent substrate scope, which is applied to synthesize over 135 amine molecules as well as a range of APIs' substructures. The crystal structure of IR-G02 reveals the determinants for altering the substrate preference. Finally, we demonstrate a gram-scale synthesis of an analogue of the API sensipar via a kinetic resolution approach, which displays ee >99%, total turnover numbers of up to 2087, and space time yield up to 18.10 g L-1 d-1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Reductive amination of ketones catalyzed by whole cell biocatalysts containing imine reductases (IREDs).J Biotechnol. 2017 Sep 20;258:167-170. doi: 10.1016/j.jbiotec.2017.05.015. Epub 2017 May 22. J Biotechnol. 2017. PMID: 28545904
-
Photometric Characterization of the Reductive Amination Scope of the Imine Reductases from Streptomyces tsukubaensis and Streptomyces ipomoeae.Chembiochem. 2017 Oct 18;18(20):2022-2027. doi: 10.1002/cbic.201700257. Epub 2017 Sep 18. Chembiochem. 2017. PMID: 28833946
-
NAD(P)H-Dependent Dehydrogenases for the Asymmetric Reductive Amination of Ketones: Structure, Mechanism, Evolution and Application.Adv Synth Catal. 2017 Jun 19;359(12):2011-2025. doi: 10.1002/adsc.201700356. Epub 2017 May 11. Adv Synth Catal. 2017. PMID: 30008635 Free PMC article. Review.
-
A Reductive Aminase Switches to Imine Reductase Mode for a Bulky Amine Substrate.ACS Catal. 2023 Jan 12;13(3):1669-1677. doi: 10.1021/acscatal.2c06066. eCollection 2023 Feb 3. ACS Catal. 2023. PMID: 36776386 Free PMC article.
-
Biocatalytic reductive aminations with NAD(P)H-dependent enzymes: enzyme discovery, engineering and synthetic applications.Chem Soc Rev. 2024 Jan 2;53(1):227-262. doi: 10.1039/d3cs00391d. Chem Soc Rev. 2024. PMID: 38059509 Review.
Cited by
-
Biosynthesis of Diverse Ephedra-Type Alkaloids via a Newly Identified Enzymatic Cascade.Biodes Res. 2024 Sep 3;6:0048. doi: 10.34133/bdr.0048. eCollection 2024. Biodes Res. 2024. PMID: 39228751 Free PMC article.
-
Discovery and Synthetic Applications of a NAD(P)H-Dependent Reductive Aminase from Rhodococcus erythropolis.ACS Catal. 2024 Dec 16;15(1):211-219. doi: 10.1021/acscatal.4c04935. eCollection 2025 Jan 3. ACS Catal. 2024. PMID: 39781332 Free PMC article.
-
Structure of the imine reductase from Ajellomyces dermatitidis in three crystal forms.Acta Crystallogr F Struct Biol Commun. 2023 Sep 1;79(Pt 9):224-230. doi: 10.1107/S2053230X23006672. Epub 2023 Aug 15. Acta Crystallogr F Struct Biol Commun. 2023. PMID: 37581897 Free PMC article.
-
Asymmetric Synthesis of 2-Arylethylamines: A Metal-Free Review of the New Millennium.Molecules. 2024 Dec 4;29(23):5729. doi: 10.3390/molecules29235729. Molecules. 2024. PMID: 39683888 Free PMC article. Review.
-
Chemoenzymatic synthesis.Commun Chem. 2025 Mar 13;8(1):77. doi: 10.1038/s42004-025-01451-z. Commun Chem. 2025. PMID: 40082686 Free PMC article.
References
-
- Jarvis LM. The year in new drugs. Chem. Eng. N. 2016;94:12–17.
-
- Ghislieri D, Turner NJ. Biocatalytic approaches to the synthesis of enantiomerically pure chiral amines. Top. Catal. 2013;57:284–300. doi: 10.1007/s11244-013-0184-1. - DOI
-
- O’Reilly E, Turner NJ. Enzymatic cascades for the regio- and stereoselective synthesis of chiral amines. Perspect. Sci. 2015;4:55–61. doi: 10.1016/j.pisc.2014.12.009. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

