Insulin-regulated serine and lipid metabolism drive peripheral neuropathy
- PMID: 36697822
- PMCID: PMC9891999
- DOI: 10.1038/s41586-022-05637-6
Insulin-regulated serine and lipid metabolism drive peripheral neuropathy
Abstract
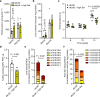
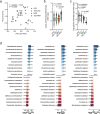
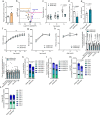
Diabetes represents a spectrum of disease in which metabolic dysfunction damages multiple organ systems including liver, kidneys and peripheral nerves1,2. Although the onset and progression of these co-morbidities are linked with insulin resistance, hyperglycaemia and dyslipidaemia3-7, aberrant non-essential amino acid (NEAA) metabolism also contributes to the pathogenesis of diabetes8-10. Serine and glycine are closely related NEAAs whose levels are consistently reduced in patients with metabolic syndrome10-14, but the mechanistic drivers and downstream consequences of this metabotype remain unclear. Low systemic serine and glycine are also emerging as a hallmark of macular and peripheral nerve disorders, correlating with impaired visual acuity and peripheral neuropathy15,16. Here we demonstrate that aberrant serine homeostasis drives serine and glycine deficiencies in diabetic mice, which can be diagnosed with a serine tolerance test that quantifies serine uptake and disposal. Mimicking these metabolic alterations in young mice by dietary serine or glycine restriction together with high fat intake markedly accelerates the onset of small fibre neuropathy while reducing adiposity. Normalization of serine by dietary supplementation and mitigation of dyslipidaemia with myriocin both alleviate neuropathy in diabetic mice, linking serine-associated peripheral neuropathy to sphingolipid metabolism. These findings identify systemic serine deficiency and dyslipidaemia as novel risk factors for peripheral neuropathy that may be exploited therapeutically.
© 2023. The Author(s).
Conflict of interest statement
C.M.M. is a scientific advisor for Faeth Therapeutics. The other authors declare no competing interests.
Figures










Comment in
-
Serine deficiency causes complications in diabetes.Nature. 2023 Feb;614(7946):42-43. doi: 10.1038/d41586-023-00054-9. Nature. 2023. PMID: 36697725 No abstract available.
-
Serine slows diabetic neuropathy in mice.Nat Rev Endocrinol. 2023 Apr;19(4):187. doi: 10.1038/s41574-023-00811-w. Nat Rev Endocrinol. 2023. PMID: 36782034 No abstract available.
-
Serine supplementation: Is it a new option for the treatment of diabetic polyneuropathy?J Diabetes Investig. 2023 Oct;14(10):1157-1159. doi: 10.1111/jdi.14047. Epub 2023 Jun 25. J Diabetes Investig. 2023. PMID: 37357504 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

