Radical hydrodifluoromethylation of unsaturated C-C bonds via an electroreductively triggered two-pronged approach
- PMID: 36697867
- PMCID: PMC9814520
- DOI: 10.1038/s42004-022-00697-1
Radical hydrodifluoromethylation of unsaturated C-C bonds via an electroreductively triggered two-pronged approach
Abstract
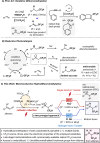
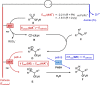
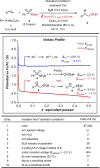
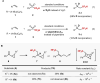
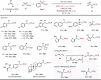
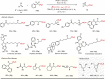
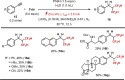
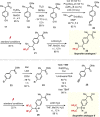
Due to its superior ability in controlling pharmaceutical activity, the installation of difluoromethyl (CF2H) functionality into organic molecules has been an area of intensive research. In this context, difluoromethylation of C-C π bonds mediated by a CF2H radical have been pursued as a central strategy to grant access to difluoromethylated hydrocarbons. However, early precedents necessitate the generation of oxidative chemical species that can limit the generality and utility of the reaction. We report here the successful implementation of radical hydrodifluoromethylation of unsaturated C-C bonds via an electroreductively triggered two-pronged approach. Preliminary mechanistic investigations suggest that the key distinction of the present strategy originates from the reconciliation of multiple redox processes under highly reducing electrochemical conditions. The reaction conditions can be chosen based on the electronic properties of the alkenes of interest, highlighting the hydrodifluoromethylation of both unactivated and activated alkenes. Notably, the reaction delivers geminal (bis)difluoromethylated products from alkynes in a single step by consecutive hydrodifluoromethylation, granting access to an underutilized 1,1,3,3-tetrafluoropropan-2-yl functional group. The late-stage hydrodifluoromethylation of densely functionalized pharmaceutical agents is also presented.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Cu-Electrocatalysis Enables Vicinal Bis(difluoromethylation) of Alkenes: Unraveling Dichotomous Role of Zn(CF2H)2(DMPU)2 as Both Radical and Anion Source.J Am Chem Soc. 2024 Aug 14;146(32):22498-22508. doi: 10.1021/jacs.4c06207. Epub 2024 Jul 30. J Am Chem Soc. 2024. PMID: 39079933
-
Copper-Catalyzed Visible-Light-Induced Allylic Difluoromethylation of Unactivated Alkenes Using Difluoroacetic Acid.Org Lett. 2023 Feb 17;25(6):1045-1049. doi: 10.1021/acs.orglett.3c00265. Epub 2023 Feb 8. Org Lett. 2023. PMID: 36752311
-
Difluoromethylated Difunctionalization of Alkenes under Visible Light.J Org Chem. 2024 Feb 16;89(4):2525-2537. doi: 10.1021/acs.joc.3c02552. Epub 2024 Feb 1. J Org Chem. 2024. PMID: 38300156
-
New synthetic approaches for the construction of difluoromethylated architectures.Org Biomol Chem. 2025 Mar 26;23(13):3029-3075. doi: 10.1039/d4ob02000f. Org Biomol Chem. 2025. PMID: 40013736 Review.
-
N-Functionalized Pyridinium Salts: A New Chapter for Site-Selective Pyridine C-H Functionalization via Radical-Based Processes under Visible Light Irradiation.Acc Chem Res. 2022 Oct 18;55(20):3043-3056. doi: 10.1021/acs.accounts.2c00530. Epub 2022 Sep 27. Acc Chem Res. 2022. PMID: 36166489 Review.
Cited by
-
A General Hydrotrifluoromethylation of Unactivated Olefins Enabled by Voltage-Gated Electrosynthesis.Angew Chem Int Ed Engl. 2025 Jan 21;64(4):e202415218. doi: 10.1002/anie.202415218. Epub 2024 Nov 11. Angew Chem Int Ed Engl. 2025. PMID: 39363774 Free PMC article.
-
Tetrafluoroisopropylation of alkenes and alkynes enabled by photocatalytic consecutive difluoromethylation with CF2HSO2Na.Nat Commun. 2024 Jul 6;15(1):5685. doi: 10.1038/s41467-024-50081-x. Nat Commun. 2024. PMID: 38971849 Free PMC article.
-
Modular synthesis of CF2-containing compounds with PhSO2CF2H reagent through difluoromethylene radical anion synthon strategy.Nat Commun. 2025 Aug 19;16(1):7731. doi: 10.1038/s41467-025-62834-3. Nat Commun. 2025. PMID: 40830095 Free PMC article.
References
Grants and funding
- KK2232-10/Korea Research Institute of Chemical Technology (KRICT)
- KK2232-10/Korea Research Institute of Chemical Technology (KRICT)
- KK2232-10/Korea Research Institute of Chemical Technology (KRICT)
- NRF-2021R1C1C1004605/National Research Foundation of Korea (NRF)
- NRF-2021R1A4A3022415/National Research Foundation of Korea (NRF)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

