Highly congested spiro-compounds via photoredox-mediated dearomative annulation cascade
- PMID: 36697909
- PMCID: PMC9814605
- DOI: 10.1038/s42004-022-00706-3
Highly congested spiro-compounds via photoredox-mediated dearomative annulation cascade
Abstract
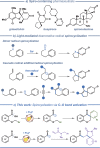
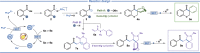
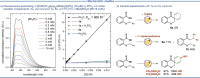
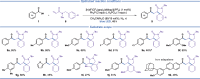
Photo-mediated radical dearomatization involving 5-exo-trig cyclizations has proven to be an important route to accessing spirocyclic compounds, whereas 6-exo-trig spirocyclization has been much less explored. In this work, a dearomative annulation cascade is realized through photoredox-mediated C-O bond activation of aromatic carboxylic acids to produce two kinds of spirocyclic frameworks. Mechanistically, the acyl radical is formed through oxidation of triphenylphosphine and subsequent C-O bond cleavage, followed by a 6-exo-trig cyclization/SET/protonation sequence to generate the spiro-chromanone products in an intramolecular manner. Furthermore, the protocol was extended to more challenging intermolecular tandem sequences consisting of C-O bond cleavage, radical addition to an alkene substrate, and 5-exo-trig cyclization to yield complex spirocyclic lactams.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Brønsted Base Assisted Photoredox Catalysis: Proton Coupled Electron Transfer for Remote C-C Bond Formation via Amidyl Radicals.Chemistry. 2018 Sep 20;24(53):14054-14058. doi: 10.1002/chem.201802907. Epub 2018 Aug 27. Chemistry. 2018. PMID: 29939456
-
Controlling the regiochemistry of radical cyclizations.Chem Rec. 2006;6(1):23-31. doi: 10.1002/tcr.20069. Chem Rec. 2006. PMID: 16470801 Review.
-
Photoredox-Catalyzed Carbamoyl Radical-Initiated Dearomative Spirocyclization To Access Spiro-Cyclohexadiene Oxindoles.Org Lett. 2024 Oct 11;26(40):8503-8508. doi: 10.1021/acs.orglett.4c03045. Epub 2024 Oct 1. Org Lett. 2024. PMID: 39353048
-
Palladium-Catalyzed Cascade Dearomative Spirocyclization and C-H Annulation of Aromatic Halides with Alkynes.Org Lett. 2021 Jun 16. doi: 10.1021/acs.orglett.1c01736. Online ahead of print. Org Lett. 2021. PMID: 34132559
-
4-Exo-Trig Radical Cyclization: A Promising Approach to Four-Membered Rings.Chemistry. 2025 Apr 9;31(21):e202500206. doi: 10.1002/chem.202500206. Epub 2025 Mar 11. Chemistry. 2025. PMID: 39996614 Review.
Cited by
-
Chemodivergent dearomatization of benzene-linked O-oxime esters via EnT-induced radical cross-coupling.Chem Sci. 2025 Jan 9;16(6):2690-2699. doi: 10.1039/d4sc07681h. eCollection 2025 Feb 5. Chem Sci. 2025. PMID: 39802692 Free PMC article.
-
Visible light-mediated dearomative spirocyclization/imination of nonactivated arenes through energy transfer catalysis.Nat Commun. 2025 Apr 16;16(1):3610. doi: 10.1038/s41467-025-58808-0. Nat Commun. 2025. PMID: 40240355 Free PMC article.
References
Grants and funding
- FZEN-2020-0022/Ministry of Education and Science of the Russian Federation (Minobrnauka)
- 2020-04764/Vetenskapsrådet (Swedish Research Council)
- 2019-01269/Svenska Forskningsrådet Formas (Swedish Research Council Formas)
- UPD2021-0145/Wenner-Gren Foundation (Wenner-Gren Foundation for Anthropological Research, Inc.)
- 204-0175/Stiftelsen Olle Engkvist Byggmästare
LinkOut - more resources
Full Text Sources

