Highly congested spiro-compounds via photoredox-mediated dearomative annulation cascade
- PMID: 36697909
- PMCID: PMC9814605
- DOI: 10.1038/s42004-022-00706-3
Highly congested spiro-compounds via photoredox-mediated dearomative annulation cascade
Abstract
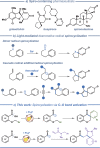
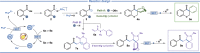
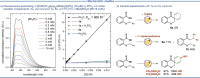
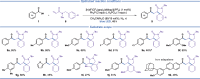
Photo-mediated radical dearomatization involving 5-exo-trig cyclizations has proven to be an important route to accessing spirocyclic compounds, whereas 6-exo-trig spirocyclization has been much less explored. In this work, a dearomative annulation cascade is realized through photoredox-mediated C-O bond activation of aromatic carboxylic acids to produce two kinds of spirocyclic frameworks. Mechanistically, the acyl radical is formed through oxidation of triphenylphosphine and subsequent C-O bond cleavage, followed by a 6-exo-trig cyclization/SET/protonation sequence to generate the spiro-chromanone products in an intramolecular manner. Furthermore, the protocol was extended to more challenging intermolecular tandem sequences consisting of C-O bond cleavage, radical addition to an alkene substrate, and 5-exo-trig cyclization to yield complex spirocyclic lactams.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Grants and funding
- FZEN-2020-0022/Ministry of Education and Science of the Russian Federation (Minobrnauka)
- 2020-04764/Vetenskapsrådet (Swedish Research Council)
- 2019-01269/Svenska Forskningsrådet Formas (Swedish Research Council Formas)
- UPD2021-0145/Wenner-Gren Foundation (Wenner-Gren Foundation for Anthropological Research, Inc.)
- 204-0175/Stiftelsen Olle Engkvist Byggmästare
LinkOut - more resources
Full Text Sources

