Manipulations of phenylnorbornyl palladium species for multicomponent construction of a bridged polycyclic privileged scaffold
- PMID: 36697919
- PMCID: PMC9814782
- DOI: 10.1038/s42004-022-00759-4
Manipulations of phenylnorbornyl palladium species for multicomponent construction of a bridged polycyclic privileged scaffold
Abstract
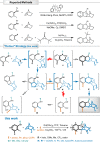
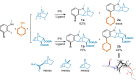
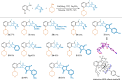
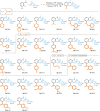
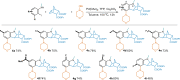
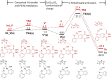
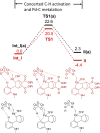
Hexahydromethanocarbazole is a privileged scaffold in the discovery of new drugs and photoactive organic materials due to its good balance between structural complexity and minimized entropy penalty upon receptor binding. To address the difficulty of synthesizing this highly desirable bridged polycyclic scaffold, we designed a convenient multicomponent reaction cascade as intercepted Heck addition/C-H activation/C-palladacycle formation/electrophilic attack of ANP/N-palladacycle formation/Buchwald amination. A distinguishing feature of this sophisticated strategy is the successive generation of two key phenylnorbornyl palladium species to control the reaction flow towards desired products. DFT calculations further reveal the crucial roles of Cs2CO3 and 5,6-diester substitutions on the norbornene reactant in preventing multiple side-reactions. This innovative method exhibits a broad scope with good yields, and therefore will enable the construction of natural-product-like compound libraries based on hexahydromethanocarbazole.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
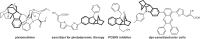

Similar articles
-
Catalytic Reactions Directed by a Structurally Well-Defined Aminomethyl Cyclopalladated Complex.Acc Chem Res. 2021 Dec 7;54(23):4305-4318. doi: 10.1021/acs.accounts.1c00365. Epub 2021 Nov 11. Acc Chem Res. 2021. PMID: 34761901
-
Catalytic sequential reactions involving palladacycle-directed aryl coupling steps.Acc Chem Res. 2008 Nov 18;41(11):1512-22. doi: 10.1021/ar800040u. Acc Chem Res. 2008. PMID: 18680317
-
A palladium/norbornene cooperative catalysis to access N-containing bridged scaffolds.Chem Commun (Camb). 2019 Jul 23;55(60):8816-8819. doi: 10.1039/c9cc03126j. Chem Commun (Camb). 2019. PMID: 31120461
-
Recent Advances in Construction of Polycyclic Natural Product Scaffolds via One-Pot Reactions Involving Alkyne Annulation.Front Chem. 2020 Oct 15;8:580355. doi: 10.3389/fchem.2020.580355. eCollection 2020. Front Chem. 2020. PMID: 33195069 Free PMC article. Review.
-
[Novel access to indazoles based on palladium-catalyzed amination chemistry].Yakugaku Zasshi. 2008 Jul;128(7):997-1005. doi: 10.1248/yakushi.128.997. Yakugaku Zasshi. 2008. PMID: 18591867 Review. Japanese.
References
Grants and funding
LinkOut - more resources
Full Text Sources

