Anti-inflammatory Therapy Protects Spiral Ganglion Neurons After Aminoglycoside Antibiotic-Induced Hair Cell Loss
- PMID: 36697994
- PMCID: PMC10121993
- DOI: 10.1007/s13311-022-01336-2
Anti-inflammatory Therapy Protects Spiral Ganglion Neurons After Aminoglycoside Antibiotic-Induced Hair Cell Loss
Abstract
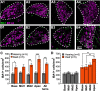
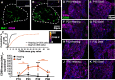
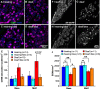
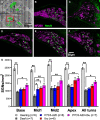
Destruction of cochlear hair cells by aminoglycoside antibiotics leads to gradual death of the spiral ganglion neurons (SGNs) that relay auditory information to the brain, potentially limiting the efficacy of cochlear implants. Because the reasons for this cochlear neurodegeneration are unknown, there are no neuroprotective strategies for patients. To investigate this problem, we assessed transcriptomic changes in the rat spiral ganglion following aminoglycoside antibiotic (kanamycin)-induced hair cell destruction. We observed selectively increased expression of immune and inflammatory response genes and increased abundance of activated macrophages in spiral ganglia by postnatal day 32 in kanamycin-deafened rats, preceding significant SGN degeneration. Treatment with the anti-inflammatory medications dexamethasone and ibuprofen diminished long-term SGN degeneration. Ibuprofen and dexamethasone also diminished macrophage activation. Efficacy of ibuprofen treatment was augmented by co-administration of the nicotinamide adenine dinucleotide-stabilizing agent P7C3-A20. Our results support a critical role of neuroinflammation in SGN degeneration after aminoglycoside antibiotic-mediated cochlear hair cell loss, as well as a neuroprotective strategy that could improve cochlear implant efficacy.
Keywords: Deafening; Dexamethasone; Ibuprofen; Inflammation; Neurodegeneration; P7C3-A20.
© 2023. The American Society for Experimental Neurotherapeutics, Inc.
Figures




References
-
- Green SH, Altschuler RA, Miller JM. Cell death and cochlear protection. In: Schacht J, Popper AN, Fay RR, editors. Auditory Trauma, Protection and Repair. Springer Handbook of Auditory Research. New York: Springer-Verlag; 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

