Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism
- PMID: 36698192
- PMCID: PMC9878808
- DOI: 10.1186/s12951-023-01788-4
Exosomes derived from human adipose mesenchymal stem cells ameliorate hepatic fibrosis by inhibiting PI3K/Akt/mTOR pathway and remodeling choline metabolism
Abstract
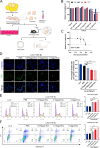
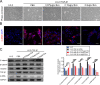
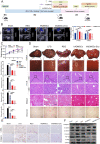
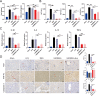
Liver fibrosis is a chronic liver disease with the presence of progressive wound healing response caused by liver injury. Currently, there are no approved therapies for liver fibrosis. Exosomes derived from human adipose mesenchymal stem cells (hADMSCs-Exo) have displayed a prominent therapeutic effect on liver diseases. However, few studies have evaluated therapeutic effect of hADMSCs-Exo in liver fibrosis and cirrhosis, and its precise mechanisms of action remain unclear. Herein, we investigated anti-fibrotic efficacy of hADMSCs-Exo in vitro and in vivo, and identified important metabolic changes and the detailed mechanism through transcriptomic and metabolomic profiling. We found hADMSCs-Exo could inhibit the proliferation of activated hepatic stellate cells through aggravating apoptosis and arresting G1 phase, effectively inhibiting the expression of profibrogenic proteins and epithelial-to-mesenchymal transition (EMT) in vitro. Moreover, it could significantly block collagen deposition and EMT process, improve liver function and reduce liver inflammation in liver cirrhosis mice model. The omics analysis revealed that the key mechanism of hADMSCs-Exo anti-hepatic fibrosis was the inhibition of PI3K/AKT/mTOR signaling pathway and affecting the changes of metabolites in lipid metabolism, and mainly regulating choline metabolism. CHPT1 activated by hADMSCs-Exo facilitated formation and maintenance of vesicular membranes. Thus, our study indicates that hADMSCs-Exo can attenuate hepatic stellate cell activation and suppress the progression of liver fibrosis, which holds the significant potential of hADMSCs-Exo for use as extracellular nanovesicles-based therapeutics in the treatment of liver fibrosis and possibly other intractable chronic liver diseases.
Keywords: Choline; Exosomes; Human adipose mesenchymal stem cells; Liver fibrosis; Metabolomics.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
ADSCs-derived exosomes ameliorate hepatic fibrosis by suppressing stellate cell activation and remodeling hepatocellular glutamine synthetase-mediated glutamine and ammonia homeostasis.Stem Cell Res Ther. 2022 Oct 4;13(1):494. doi: 10.1186/s13287-022-03049-x. Stem Cell Res Ther. 2022. PMID: 36195966 Free PMC article.
-
Comprehensive evaluation of the mechanism of human adipose mesenchymal stem cells ameliorating liver fibrosis by transcriptomics and metabolomics analysis.Sci Rep. 2024 Aug 28;14(1):20035. doi: 10.1038/s41598-024-70281-1. Sci Rep. 2024. PMID: 39198546 Free PMC article.
-
Ameliorative effects of HGF-overexpressed exosomes derived from ADMSCs on oxidative stress in hepatic fibrosis.Histol Histopathol. 2025 May;40(5):757-772. doi: 10.14670/HH-18-816. Epub 2024 Sep 18. Histol Histopathol. 2025. PMID: 39397450
-
A systematic review of the anti-inflammatory and anti-fibrotic potential of human umbilical cord mesenchymal stem cells-derived exosomes in experimental models of liver regeneration.Mol Biol Rep. 2024 Sep 20;51(1):999. doi: 10.1007/s11033-024-09929-0. Mol Biol Rep. 2024. PMID: 39302506
-
Progress of mesenchymal stem cells (MSCs) & MSC-Exosomes combined with drugs intervention in liver fibrosis.Biomed Pharmacother. 2024 Jul;176:116848. doi: 10.1016/j.biopha.2024.116848. Epub 2024 Jun 3. Biomed Pharmacother. 2024. PMID: 38834005 Review.
Cited by
-
Immune microenvironment changes of liver cirrhosis: emerging role of mesenchymal stromal cells.Front Immunol. 2023 Jul 19;14:1204524. doi: 10.3389/fimmu.2023.1204524. eCollection 2023. Front Immunol. 2023. PMID: 37539053 Free PMC article. Review.
-
Hotspots and trends in stem cell therapy for liver fibrosis and cirrhosis: A bibliometric analysis.World J Hepatol. 2025 Jan 27;17(1):96105. doi: 10.4254/wjh.v17.i1.96105. World J Hepatol. 2025. PMID: 39871895 Free PMC article.
-
Single-cell RNA-seq uncovers lineage-specific regulatory alterations of fibroblasts and endothelial cells in ligamentum flavum hypertrophy.Front Immunol. 2025 May 15;16:1569296. doi: 10.3389/fimmu.2025.1569296. eCollection 2025. Front Immunol. 2025. PMID: 40443657 Free PMC article.
-
Review of Chinese medicine intervention in PI3K/AKT pathway to regulate fibrosis.Medicine (Baltimore). 2025 Jul 11;104(28):e42957. doi: 10.1097/MD.0000000000042957. Medicine (Baltimore). 2025. PMID: 40660593 Free PMC article. Review.
-
A new insight: crosstalk between neutrophil extracellular traps and the gut-liver axis for nonalcoholic fatty liver disease.Front Immunol. 2025 Jun 27;16:1599956. doi: 10.3389/fimmu.2025.1599956. eCollection 2025. Front Immunol. 2025. PMID: 40655142 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- 2018HH0062/Sichuan Province Science and Technology Support Program
- 2021YFH0187/Sichuan Science and Technology Program
- ZYGX2021YGCX018/Medico-Engineering Cooperation Funds from University of Electronic Science and Technology of China
- ZYGX2020KYQD002/Fundamental Research Funds for the Central Universities
LinkOut - more resources
Full Text Sources
Miscellaneous

