Nicotinamide Mononucleotide Ameliorates Sleep Deprivation-Induced Gut Microbiota Dysbiosis and Restores Colonization Resistance against Intestinal Infections
- PMID: 36698264
- PMCID: PMC10037695
- DOI: 10.1002/advs.202207170
Nicotinamide Mononucleotide Ameliorates Sleep Deprivation-Induced Gut Microbiota Dysbiosis and Restores Colonization Resistance against Intestinal Infections
Abstract
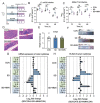
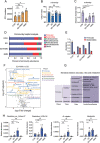
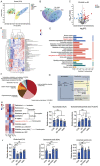
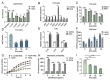
Gut microbiota-mediated colonization resistance (CR) is crucial in protecting the host from intestinal infections. Sleep deprivation (SD) is an important contributor in the disturbances of intestinal homeostasis. However, whether and how SD affects host CR remains largely unknown. Here, it is shown that SD impairs intestinal CR in mice, whereas nicotinamide mononucleotide (NMN) supplementation restores it. Microbial diversity and metabolomic analyses suggest that gut microbiota and metabolite profiles in SD-treated mice are highly shaped, whereas NMN reprograms these differences. Specifically, the altered gut microbiota in SD mice further incurs the disorder of secondary bile acids pool accompanied by a decrease in deoxycholic acid (DCA). Conversely, NMN supplementation retakes the potential benefits of DCA, which is associated with specific gut microbiota involved in primary bile acids metabolic flux. In animal models of infection, DCA is effective in preventing and treating bacterial infections when used alone or in combination with antibiotics. Mechanistically, DCA alone disrupts membrane permeability and aggravates oxidative damage, thereby reducing intestinal pathogen burden. Meanwhile, exogenous DCA promotes antibiotic accumulation and destroys oxidant-antioxidant system, thus potentiating antibiotic efficacy. Overall, this work highlights the important roles of gut microbiota and bile acid metabolism in the maintenance of intestinal CR.
Keywords: colonization resistance; gut microbiota; intestinal infections; metabolites; sleep deprivation.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Modulation of the Gut Microbiota-farnesoid X Receptor Axis Improves Deoxycholic Acid-induced Intestinal Inflammation in Mice.J Crohns Colitis. 2021 Jul 5;15(7):1197-1210. doi: 10.1093/ecco-jcc/jjab003. J Crohns Colitis. 2021. PMID: 33417675
-
NMN alleviates radiation-induced intestinal fibrosis by modulating gut microbiota.Int J Radiat Biol. 2023;99(5):823-834. doi: 10.1080/09553002.2023.2145029. Epub 2022 Nov 16. Int J Radiat Biol. 2023. PMID: 36343364
-
Alcohol-induced gut microbiome dysbiosis enhances the colonization of Klebsiella pneumoniae on the mouse intestinal tract.mSystems. 2024 Mar 19;9(3):e0005224. doi: 10.1128/msystems.00052-24. Epub 2024 Feb 12. mSystems. 2024. PMID: 38345382 Free PMC article.
-
Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex.J Autoimmun. 2018 Aug;92:12-34. doi: 10.1016/j.jaut.2018.05.008. Epub 2018 Jun 1. J Autoimmun. 2018. PMID: 29861127 Review.
-
The Role of the Gut Microbiota in Bile Acid Metabolism.Ann Hepatol. 2017 Nov;16(Suppl. 1: s3-105.):s15-s20. doi: 10.5604/01.3001.0010.5494. Ann Hepatol. 2017. PMID: 29080339 Review.
Cited by
-
Microbiota metabolites affect sleep as drivers of brain‑gut communication (Review).Int J Mol Med. 2025 Sep;56(3):130. doi: 10.3892/ijmm.2025.5571. Epub 2025 Jul 4. Int J Mol Med. 2025. PMID: 40613226 Free PMC article. Review.
-
Bacillus siamensis Improves the Immune Status and Intestinal Health of Weaned Piglets by Improving Their Intestinal Microbiota.Microorganisms. 2024 May 17;12(5):1012. doi: 10.3390/microorganisms12051012. Microorganisms. 2024. PMID: 38792841 Free PMC article.
-
Advancements in NMN biotherapy and research updates in the field of digestive system diseases.J Transl Med. 2024 Aug 30;22(1):805. doi: 10.1186/s12967-024-05614-9. J Transl Med. 2024. PMID: 39215316 Free PMC article. Review.
-
Dietary Fiber-Derived Microbial Butyrate Suppresses ILC2-Dependent Airway Inflammation in COPD.Mediators Inflamm. 2024 Jul 9;2024:6263447. doi: 10.1155/2024/6263447. eCollection 2024. Mediators Inflamm. 2024. PMID: 39015676 Free PMC article.
-
Faecalibacterium prausnitzii Supplementation Prevents Intestinal Barrier Injury and Gut Microflora Dysbiosis Induced by Sleep Deprivation.Nutrients. 2024 Apr 9;16(8):1100. doi: 10.3390/nu16081100. Nutrients. 2024. PMID: 38674791 Free PMC article.
References
-
- Kaplan G. G., Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2021YFD1801000/National Key Research and Development Program of China
- 2018YFA0903400/National Key Research and Development Program of China
- 32222084/National Natural Science Foundation of China
- 32172907/National Natural Science Foundation of China
- 32002331/National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
