Ionic Conduction in Polymer-Based Solid Electrolytes
- PMID: 36698303
- PMCID: PMC10074084
- DOI: 10.1002/advs.202201718
Ionic Conduction in Polymer-Based Solid Electrolytes
Abstract
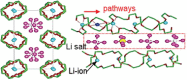
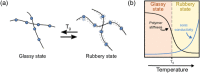
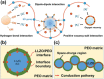
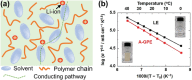
Good safety, high interfacial compatibility, low cost, and facile processability make polymer-based solid electrolytes promising materials for next-generation batteries. Key issues related to polymer-based solid electrolytes, such as synthesis methods, ionic conductivity, and battery architecture, are investigated in past decades. However, mechanistic understanding of the ionic conduction is still lacking, which impedes the design and optimization of polymer-based solid electrolytes. In this review, the ionic conduction mechanisms and optimization strategies of polymer-based solid electrolytes, including solvent-free polymer electrolytes, composite polymer electrolytes, and quasi-solid/gel polymer electrolytes, are summarized and evaluated. Challenges and strategies for enhancing the ionic conductivity are elaborated, while the ion-pair dissociation, ion mobility, polymer relaxation, and interactions at polymer/filler interfaces are highlighted. This comprehensive review is especially pertinent for the targeted enhancement of the Li-ion conductivity of polymer-based solid electrolytes.
Keywords: composite polymer electrolyte; interfacial interaction; ionic conduction; polymer electrolyte; solid-state batteries.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- a) Hu Y.‐S., Nat. Energy 2016, 1, 16042;
- b) Janek J., Zeier W. G., Nat. Energy 2016, 1, 16141;
- c) Manthiram A., Yu X., Wang S., Nat. Rev. Mater. 2017, 2, 16103;
- d) Zhang L., Yang T., Du C., Liu Q., Tang Y., Zhao J., Wang B., Chen T., Sun Y., Jia P., Li H., Geng L., Chen J., Ye H., Wang Z., Li Y., Sun H., Li X., Dai Q., Tang Y., Peng Q., Shen T., Zhang S., Zhu T., Huang J., Nat. Nanotechnol. 2020, 15, 94; - PubMed
- e) Motavalli J., Nature 2015, 526, S96. - PubMed
-
- a) Yang C., Fu K., Zhang Y., Hitz E., Hu L., Adv. Mater. 2017, 29, 1701169; - PubMed
- b) Liu B., Zhang J.‐G., Xu W., Joule 2018, 2, 833;
- c) Cheng X.‐B., Zhao C.‐Z., Yao Y.‐X., Liu H., Zhang Q., Chem 2019, 5, 74;
- d) Xia S., Wu X., Zhang Z., Cui Y., Liu W., Chem 2019, 5, 753.
-
- a) Wang X., Kerr R., Chen F., Goujon N., Pringle J. M., Mecerreyes D., Forsyth M., Howlett P. C., Adv. Mater. 2020, 32, 1905219; - PubMed
- b) Wang C., Fu K., Kammampata S. P., McOwen D. W., Samson A. J., Zhang L., Hitz G. T., Nolan A. M., Wachsman E. D., Mo Y., Thangadurai V., Hu L., Chem. Rev. 2020, 120, 4257; - PubMed
- c) Long L., Wang S., Xiao M., Meng Y., J. Mater. Chem. A 2016, 4, 10038.
-
- Zhao Q., Stalin S., Zhao C.‐Z., Archer L. A., Nat. Rev. Mater. 2020, 5, 229.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
