Ferroportin depletes iron needed for cell cycle progression in head and neck squamous cell carcinoma
- PMID: 36698390
- PMCID: PMC9868905
- DOI: 10.3389/fonc.2022.1025434
Ferroportin depletes iron needed for cell cycle progression in head and neck squamous cell carcinoma
Abstract
Introduction: Ferroportin (FPN), the only identified eukaryotic iron efflux channel, plays an important role in iron homeostasis and is downregulated in many cancers. To determine if iron related pathways are important for Head and Neck Squamous Cell Carcinoma (HNSCC) progression and proliferation, we utilize a model of FPN over-expression to simulate iron depletion and probe associated molecular pathways.
Methods: The state of iron related proteins and ferroptosis sensitivity was assessed in a panel of metastatic HNSCC cell lines. Stable, inducible expression of FPN was confirmed in the metastatic HNSCC lines HN12 and JHU-022 as well as the non-transformed normal oral keratinocyte (NOK) cell line and the effect of FPN mediated iron depletion was assessed in these cell lines.
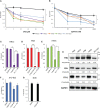
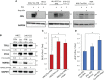
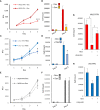
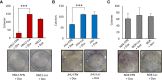
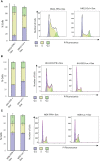
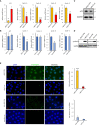
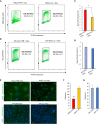
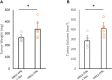
Results: HNSCC cells are sensitive to iron chelation and ferroptosis, but the non-transformed NOK cell line is not. We found that FPN expression inhibits HNSCC cell proliferation and colony formation but NOK cells are unaffected. Inhibition of cell proliferation is rescued by the addition of hepcidin. Decreases in proliferation are due to the disruption of iron homeostasis via loss of labile iron caused by elevated FPN levels. This in turn protects HNSCC cells from ferroptotic cell death. Expression of FPN induces DNA damage, activates p21, and reduces levels of cyclin proteins thereby inhibiting cell cycle progression of HNSCC cells, arresting cells in the S-phase. Induction of FPN severely inhibits Edu incorporation and increased β-galactosidase activity, indicating cells have entered senescence. Finally, in an oral orthotopic mouse xenograft model, FPN induction yields a significant decrease in tumor growth.
Conclusions: Our results indicate that iron plays a role in HNSCC cell proliferation and growth and is important for cell cycle progression. Iron based interventional strategies such as ferroptosis or iron chelation may have potential therapeutic benefits in advanced HNSCC.
Keywords: Oral Epithelial Cells; cell proliferation; ferroportin; head and neck squamous cell carcinoma; iron metabolism; iron/cell proliferation; senescence.
Copyright © 2023 Belvin and Lewis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Research progress on ferroptosis in head and neck squamous cell carcinoma.J Mol Histol. 2025 Mar 17;56(2):109. doi: 10.1007/s10735-025-10381-y. J Mol Histol. 2025. PMID: 40095205 Review.
-
Effects of Ferroportin-Mediated Iron Depletion in Cells Representative of Different Histological Subtypes of Prostate Cancer.Antioxid Redox Signal. 2019 Mar 10;30(8):1043-1061. doi: 10.1089/ars.2017.7023. Epub 2017 Dec 11. Antioxid Redox Signal. 2019. PMID: 29061069 Free PMC article.
-
Ferroportin-Dependent Iron Homeostasis Protects against Oxidative Stress-Induced Nucleus Pulposus Cell Ferroptosis and Ameliorates Intervertebral Disc Degeneration In Vivo.Oxid Med Cell Longev. 2021 Feb 10;2021:6670497. doi: 10.1155/2021/6670497. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33628376 Free PMC article.
-
miR-20a regulates expression of the iron exporter ferroportin in lung cancer.J Mol Med (Berl). 2016 Mar;94(3):347-59. doi: 10.1007/s00109-015-1362-3. Epub 2015 Nov 12. J Mol Med (Berl). 2016. PMID: 26560875 Free PMC article.
-
The role of ferroptosis in radiotherapy and combination therapy for head and neck squamous cell carcinoma (Review).Oncol Rep. 2024 Jun;51(6):79. doi: 10.3892/or.2024.8738. Epub 2024 Apr 19. Oncol Rep. 2024. PMID: 38639185 Free PMC article. Review.
Cited by
-
Transferrin receptor uptakes iron from tumor-associated neutrophils to regulate invasion patterns of OSCC.Cancer Immunol Immunother. 2025 Jan 3;74(2):43. doi: 10.1007/s00262-024-03894-0. Cancer Immunol Immunother. 2025. PMID: 39751915 Free PMC article.
-
Research progress on ferroptosis in head and neck squamous cell carcinoma.J Mol Histol. 2025 Mar 17;56(2):109. doi: 10.1007/s10735-025-10381-y. J Mol Histol. 2025. PMID: 40095205 Review.
-
Iron Metabolism in Cancer and Senescence: A Cellular Perspective.Biology (Basel). 2023 Jul 11;12(7):989. doi: 10.3390/biology12070989. Biology (Basel). 2023. PMID: 37508419 Free PMC article. Review.
-
Iron, Ferroptosis, and Head and Neck Cancer.Int J Mol Sci. 2023 Oct 12;24(20):15127. doi: 10.3390/ijms242015127. Int J Mol Sci. 2023. PMID: 37894808 Free PMC article. Review.
-
New insights into RAS in head and neck cancer.Biochim Biophys Acta Rev Cancer. 2023 Nov;1878(6):188963. doi: 10.1016/j.bbcan.2023.188963. Epub 2023 Aug 22. Biochim Biophys Acta Rev Cancer. 2023. PMID: 37619805 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

