Microbiological safety of aged meat
- PMID: 36698487
- PMCID: PMC9850206
- DOI: 10.2903/j.efsa.2023.7745
Microbiological safety of aged meat
Abstract
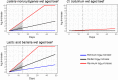
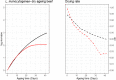
The impact of dry-ageing of beef and wet-ageing of beef, pork and lamb on microbiological hazards and spoilage bacteria was examined and current practices are described. As 'standard fresh' and wet-aged meat use similar processes these were differentiated based on duration. In addition to a description of the different stages, data were collated on key parameters (time, temperature, pH and aw) using a literature survey and questionnaires. The microbiological hazards that may be present in all aged meats included Shiga toxin-producing Escherichia coli (STEC), Salmonella spp., Staphylococcus aureus, Listeria monocytogenes, enterotoxigenic Yersinia spp., Campylobacter spp. and Clostridium spp. Moulds, such as Aspergillus spp. and Penicillium spp., may produce mycotoxins when conditions are favourable but may be prevented by ensuring a meat surface temperature of -0.5 to 3.0°C, with a relative humidity (RH) of 75-85% and an airflow of 0.2-0.5 m/s for up to 35 days. The main meat spoilage bacteria include Pseudomonas spp., Lactobacillus spp. Enterococcus spp., Weissella spp., Brochothrix spp., Leuconostoc spp., Lactobacillus spp., Shewanella spp. and Clostridium spp. Under current practices, the ageing of meat may have an impact on the load of microbiological hazards and spoilage bacteria as compared to standard fresh meat preparation. Ageing under defined and controlled conditions can achieve the same or lower loads of microbiological hazards and spoilage bacteria than the variable log10 increases predicted during standard fresh meat preparation. An approach was used to establish the conditions of time and temperature that would achieve similar or lower levels of L. monocytogenes and Yersinia enterocolitica (pork only) and lactic acid bacteria (representing spoilage bacteria) as compared to standard fresh meat. Finally, additional control activities were identified that would further assure the microbial safety of dry-aged beef, based on recommended best practice and the outputs of the equivalence assessment.
Keywords: Meat; bacterial growth; dry‐ageing; maturation; safety; wet‐ageing.
© 2023 Wiley‐VCH Verlag GmbH & Co. KgaA on behalf of the European Food Safety Authority.
Figures

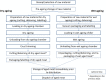

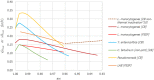
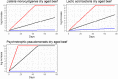


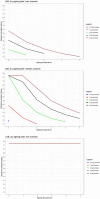

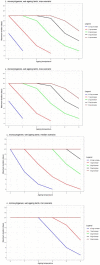
Data from: (Lee et al., 2014); (Wang et al., 2015); (Solomakos et al., 2008); (Giménez et al., 2021) ComBase records ID: M371_LM; M372_LM; M373_LM; M374_LM; M781_LM; M801_LM; M754_LM; M782_LM; M755_LM; M905_LM; M783_LM; M803_LM; M53_LM; M798_LM; M756_LM; M804_LM; M369_LM; M370_LM; M757_LM; M52_LM; M758_LM; M759_LM; M760_LM; M761_LM; M784_LM; M805_LM; M785_LM; M762_LM; M763_LM; M779_LM; M786_LM; M807_LM; M764_LM; M787_LM; M799_LM; M765_LM; M766_LM; M788_LM; M789_LM; M808_LM; M767_LM; M768_LM; M769_LM; M770_LM; M771_LM; M790_LM; M791_LM; M800_LM; M809_LM; M772_LM; M773_LM; M774_LM; M775_LM; M776_LM; M792_LM; M793_LM; M777_LM; M778_LM; M780_LM; M794_LM; M795_LM; Pawr_1.
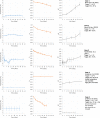
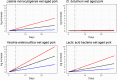
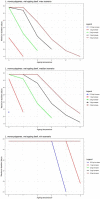
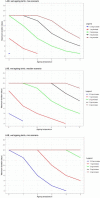
Data from: (Barrera et al., 2007) Barrera et al. (2007); IRTA unpublished data; ComBase records ID: L‐1MRSA1; L‐1MRSA2; L‐1MRSAn1; L‐1MRSAn2; L2MRSA1; L2MRSA2; L2MRSAn1; L2MRSAn2; L7MRSA1; L7MRSA2; L7MRSAn1; L7MRSAn2; L_LAB0_1; L_LAB0_2; L_LAB‐1.5_1; L_LAB2_1; L_LAB2_2; L_LAB4_1; L_LAB4_2; L_LAB7_1; S_LAB0_1; S_LAB‐1.5_1; S_LAB2_1; S_LAB2_2; S_LAB4_1; S_LAB7_1; S_LAB7_2; CB01k_1_12; CB02k_1_12; CB03k_1_12; CB04k_1_12; CB05k_1_12; CB05k_2_12; CB05k_3_12; CB06k_1_12; CB06k_2_12; CB06k_3_12; CB07k_1_12; CB07k_2_12; CB07k_3_12; CB08k_1_12; CB08k_2_12; CB08k_3_12.

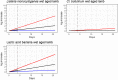
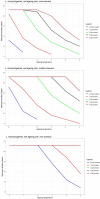
Data from Skandamis and Nychas (2002a,b); Koutsoumanis et al. (2006); Tsigarida and Nychas (2001); Delaquis and McCurdy (1990); Lasta et al. (1995); Skandamis and Nychas (2002a,b) and ComBase records ID: allP_78; allP_79; allP_80; NP_101; NP_93; NP_97; NP_102; NP_94; NP_98; NP_103; NP_95; NP_99; NP_100; NP_104; NP_96; KTM_13; KTC_17; KTC_21; KTC_25; KTC_29; KMS_11; KML_11; Tas3484; Tas3485; Tas3486; Tas3487; Tas3499; Tas3500; Barrera_Ps1; PA_16; PA_15; PA_14; PA_13; BA_28; BA_27; BA_26; BA_25; PA_40; PA_39; PA_38; PA_37; BA_44; BA_43; BA_42; BA_41; PA_52; PA_51; PA_50.

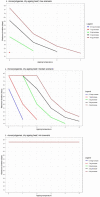
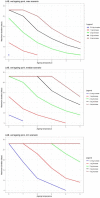
Data from Gill and Reichel (1989); Bodnaruk and Draughon (1998); Özbaş et al. (1996) and ComBase records ID: Ye03_low_4; Ye03_high_4; Ye09_low_4; Ye09_high_4; Ye03_low_10; Ye03_high_10; Ye09_low_10; Ye09_high_10; Ye03_low_15; Ye03_high_15; Ye09_low_15; Ye09_high_15; M53_Ye; M54_Ye; M52_Ye; YE1_AIR_4; YE2_AIR_4; YE1_VP_4; YE2_VP_4.
Similar articles
-
Microbiological safety of dry-aged meat: a critical review of data gaps and research needs to define process hygiene and safety criteria.Ital J Food Saf. 2024 May 13;13(3):12438. doi: 10.4081/ijfs.2024.12438. eCollection 2024 Aug 5. Ital J Food Saf. 2024. PMID: 39233700 Free PMC article. Review.
-
Bacterial spoilage of meat and cured meat products.Int J Food Microbiol. 1996 Nov;33(1):103-20. doi: 10.1016/0168-1605(96)01135-x. Int J Food Microbiol. 1996. PMID: 8913812 Review.
-
Viability of Shiga Toxin-Producing Escherichia coli, Salmonella spp., and Listeria monocytogenes during Preparation and Storage of Fuet, a Traditional Dry-Cured Spanish Pork Sausage.J Food Prot. 2022 May 1;85(5):879-889. doi: 10.4315/JFP-21-356. J Food Prot. 2022. PMID: 35294002
-
How a routine checking of Escherichia coli in retailed food of animal origin can protect consumers against exposition to Campylobacter spp. and Listeria monocytogenes?Vojnosanit Pregl. 2010 Aug;67(8):627-33. doi: 10.2298/vsp1008627t. Vojnosanit Pregl. 2010. PMID: 20845664
-
The microbiology of beef carcasses and primals during chilling and commercial storage.Food Microbiol. 2017 Feb;61:50-57. doi: 10.1016/j.fm.2016.08.003. Epub 2016 Aug 16. Food Microbiol. 2017. PMID: 27697169
Cited by
-
Microbiological safety of dry-aged meat: a critical review of data gaps and research needs to define process hygiene and safety criteria.Ital J Food Saf. 2024 May 13;13(3):12438. doi: 10.4081/ijfs.2024.12438. eCollection 2024 Aug 5. Ital J Food Saf. 2024. PMID: 39233700 Free PMC article. Review.
-
In-depth characterization of food and environmental microbiomes across different meat processing plants.Microbiome. 2024 Oct 15;12(1):199. doi: 10.1186/s40168-024-01856-3. Microbiome. 2024. PMID: 39407346 Free PMC article.
-
Effect of Dry Aging of Pork on Microbiological Quality and Instrumental Characteristics.Foods. 2024 Sep 25;13(19):3037. doi: 10.3390/foods13193037. Foods. 2024. PMID: 39410073 Free PMC article.
-
Lactic acid bacteria: beyond fermentation to bio-protection against fungal spoilage and mycotoxins in food systems.Front Microbiol. 2025 Jun 30;16:1580670. doi: 10.3389/fmicb.2025.1580670. eCollection 2025. Front Microbiol. 2025. PMID: 40661977 Free PMC article. Review.
-
Reduction of the microbial load in meat maturation rooms with and without alkaline electrolyzed water fumigation.Ital J Food Saf. 2023 Aug 1;12(3):11109. doi: 10.4081/ijfs.2023.11109. eCollection 2023 Aug 2. Ital J Food Saf. 2023. PMID: 37822575 Free PMC article.
References
-
- ACMSF , 2020. The safety and shelf‐life of vacuum and modified atmosphere packed chilled foods with respect to non‐proteolytic Clostridium botulinum . Acta Biologica Szegediensis. Available online: http://www2.sci.u-szeged.hu/ABS
-
- Adam KH, Flint SH and Brightwell G, 2010. Psychrophilic and psychrotrophic clostridia: sporulation and germination processes and their role in the spoilage of chilled, vacuum‐packaged beef, lamb and venison. International Journal of Food Science and Technology, 45, 1539–1544. 10.1111/j.1365-2621.2010.02320.x - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
