Twenty-five years of clinical applications using adaptive optics ophthalmoscopy [Invited]
- PMID: 36698659
- PMCID: PMC9841996
- DOI: 10.1364/BOE.472274
Twenty-five years of clinical applications using adaptive optics ophthalmoscopy [Invited]
Abstract
Twenty-five years ago, adaptive optics (AO) was combined with fundus photography, thereby initiating a new era in the field of ophthalmic imaging. Since that time, clinical applications of AO ophthalmoscopy to investigate visual system structure and function in both health and disease abound. To date, AO ophthalmoscopy has enabled visualization of most cell types in the retina, offered insight into retinal and systemic disease pathogenesis, and been integrated into clinical trials. This article reviews clinical applications of AO ophthalmoscopy and addresses remaining challenges for AO ophthalmoscopy to become fully integrated into standard ophthalmic care.
© 2022 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
JIWM is a co-inventor on US Patent 8226236 and US Patent App. 16/389,942 and receives funding from AGTC. TYPC: none. KG is a co-founder of SharpEye.
Figures



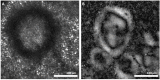
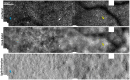

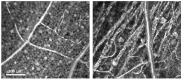


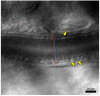




References
-
- Lu R., Aguilera N., Liu T., Liu J., Giannini J. P., Li J., Bower A. J., Dubra A., Tam J., “In-vivo sub-diffraction adaptive optics imaging of photoreceptors in the human eye with annular pupil illumination and sub-Airy detection,” Optica 8(3), 333–343 (2021).10.1364/OPTICA.414206 - DOI - PMC - PubMed
-
- Marcos S., Werner J. S., Burns S. A., Merigan W. H., Artal P., Atchison D. A., Hampson K. M., Legras R., Lundstrom L., Yoon G., Carroll J., Choi S. S., Doble N., Dubis A. M., Dubra A., Elsner A., Jonnal R., Miller D. T., Paques M., Smithson H. E., Young L. K., Zhang Y., Campbell M., Hunter J., Metha A., Palczewska G., Schallek J., Sincich L. C., “Vision science and adaptive optics, the state of the field,” Vision Res. 132, 3–33 (2017).10.1016/j.visres.2017.01.006 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
