Recent advances in optical elastography and emerging opportunities in the basic sciences and translational medicine [Invited]
- PMID: 36698669
- PMCID: PMC9842001
- DOI: 10.1364/BOE.468932
Recent advances in optical elastography and emerging opportunities in the basic sciences and translational medicine [Invited]
Abstract
Optical elastography offers a rich body of imaging capabilities that can serve as a bridge between organ-level medical elastography and single-molecule biophysics. We review the methodologies and recent developments in optical coherence elastography, Brillouin microscopy, optical microrheology, and photoacoustic elastography. With an outlook toward maximizing the basic science and translational clinical impact of optical elastography technologies, we discuss potential ways that these techniques can integrate not only with each other, but also with supporting technologies and capabilities in other biomedical fields. By embracing cross-modality and cross-disciplinary interactions with these parallel fields, optical elastography can greatly increase its potential to drive new discoveries in the biomedical sciences as well as the development of novel biomechanics-based clinical diagnostics and therapeutics.
© 2022 Optica Publishing Group under the terms of the Optica Open Access Publishing Agreement.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures






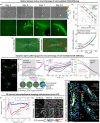
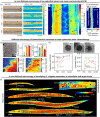
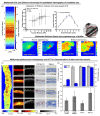

References
Publication types
LinkOut - more resources
Full Text Sources
