Luteolin-rich fraction from Perilla frutescens seed meal inhibits spike glycoprotein S1 of SARS-CoV-2-induced NLRP3 inflammasome lung cell inflammation via regulation of JAK1/STAT3 pathway: A potential anti-inflammatory compound against inflammation-induced long-COVID
- PMID: 36698809
- PMCID: PMC9870545
- DOI: 10.3389/fmed.2022.1072056
Luteolin-rich fraction from Perilla frutescens seed meal inhibits spike glycoprotein S1 of SARS-CoV-2-induced NLRP3 inflammasome lung cell inflammation via regulation of JAK1/STAT3 pathway: A potential anti-inflammatory compound against inflammation-induced long-COVID
Abstract
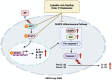
Objective: The multi-systemic inflammation as a result of COVID-19 can persevere long after the initial symptoms of the illness have subsided. These effects are referred to as Long-COVID. Our research focused on the contribution of the Spike protein S1 subunit of SARS-CoV-2 (Spike S1) on the lung inflammation mediated by NLRP3 inflammasome machinery and the cytokine releases, interleukin 6 (IL-6), IL-1beta, and IL-18, in lung epithelial cells. This study has attempted to identify the naturally- occurring agents that act against inflammation-related long-COVID. The seed meal of Perilla frutescens (P. frutescens), which contains two major dietary polyphenols (rosmarinic acid and luteolin), has been reported to exhibit anti-inflammation activities. Therefore, we have established the ethyl acetate fraction of P. frutescens seed meal (PFEA) and determined its anti-inflammatory effects on Spike S1 exposure in A549 lung cells.
Methods: PFEA was established using solvent-partitioned extraction. Rosmarinic acid (Ra) and luteolin (Lu) in PFEA were identified using the HPLC technique. The inhibitory effects of PFEA and its active compounds against Spike S1-induced inflammatory response in A549 cells were determined by RT-PCR and ELISA. The mechanistic study of anti-inflammatory properties of PFEA and Lu were determined using western blot technique.
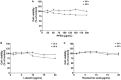
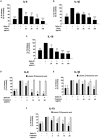
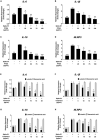
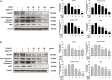
Results: PFEA was found to contain Ra (388.70 ± 11.12 mg/g extract) and Lu (248.82 ± 12.34 mg/g extract) as its major polyphenols. Accordingly, A549 lung cells were pre-treated with PFEA (12.5-100 μg/mL) and its two major compounds (2.5-20 μg/mL) prior to the Spike S1 exposure at 100 ng/mL. PFEA dose-dependently exhibited anti-inflammatory properties upon Spike S1-exposed A549 cells through IL-6, IL-1β, IL-18, and NLRP3 gene suppressions, as well as IL-6, IL-1β, and IL-18 cytokine releases with statistical significance (p < 0.05). Importantly, Lu possesses superior anti-inflammatory properties when compared with Ra (p < 0.01). Mechanistically, PFEA and Lu effectively attenuated a Spike S1-induced inflammatory response through downregulation of the JAK1/STAT3-inflammasome-dependent inflammatory pathway as evidenced by the downregulation of NLRP3, ASC, and cleaved-caspase-1 of the NLRP3 inflammasome components and by modulating the phosphorylation of JAK1 and STAT3 proteins (p < 0.05).
Conclusion: The findings suggested that luteolin and PFEA can modulate the signaling cascades that regulate Spike S1-induced lung inflammation during the incidence of Long-COVID. Consequently, luteolin and P. frutescens may be introduced as potential candidates in the preventive therapeutic strategy for inflammation-related post-acute sequelae of COVID-19.
Keywords: JAK1/STAT3 pathway; NLRP3 inflammasome pathway; Perilla frutescens extract; anti-inflammation; long-COVID; lung inflammation; luteolin (Lu); spike glycoprotein S1.
Copyright © 2023 Dissook, Umsumarng, Mapoung, Semmarath, Arjsri, Srisawad and Dejkriengkraikul.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Luteolin-Rich Extract from Harrisonia perforata (Blanco) Merr. Root Alleviates SARS-CoV-2 Spike Protein-Stimulated Lung Inflammation via Inhibition of MAPK/NLRP3 Inflammasome Signaling Pathways.Life (Basel). 2025 Jul 5;15(7):1077. doi: 10.3390/life15071077. Life (Basel). 2025. PMID: 40724579 Free PMC article.
-
Hesperetin from Root Extract of Clerodendrum petasites S. Moore Inhibits SARS-CoV-2 Spike Protein S1 Subunit-Induced NLRP3 Inflammasome in A549 Lung Cells via Modulation of the Akt/MAPK/AP-1 Pathway.Int J Mol Sci. 2022 Sep 7;23(18):10346. doi: 10.3390/ijms231810346. Int J Mol Sci. 2022. PMID: 36142258 Free PMC article.
-
Cyanidin-3-O-glucoside and Peonidin-3-O-glucoside-Rich Fraction of Black Rice Germ and Bran Suppresses Inflammatory Responses from SARS-CoV-2 Spike Glycoprotein S1-Induction In Vitro in A549 Lung Cells and THP-1 Macrophages via Inhibition of the NLRP3 Inflammasome Pathway.Nutrients. 2022 Jun 30;14(13):2738. doi: 10.3390/nu14132738. Nutrients. 2022. PMID: 35807916 Free PMC article.
-
COVID-19: A Case for Inhibiting NLRP3 Inflammasome, Suppression of Inflammation with Curcumin?Basic Clin Pharmacol Toxicol. 2021 Jan;128(1):37-45. doi: 10.1111/bcpt.13503. Epub 2020 Oct 24. Basic Clin Pharmacol Toxicol. 2021. PMID: 33099890 Review.
-
[Progress in the relationship between NLRP3 inflammasome and lung injury in COVID-19].Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2021 Sep;37(9):844-850. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2021. PMID: 34533131 Review. Chinese.
Cited by
-
Vitamin D regulates COVID-19 associated severity by suppressing the NLRP3 inflammasome pathway.PLoS One. 2024 May 15;19(5):e0302818. doi: 10.1371/journal.pone.0302818. eCollection 2024. PLoS One. 2024. PMID: 38748756 Free PMC article.
-
Anti-Inflammatory and Neuroprotective Polyphenols Derived from the European Olive Tree, Olea europaea L., in Long COVID and Other Conditions Involving Cognitive Impairment.Int J Mol Sci. 2024 Oct 14;25(20):11040. doi: 10.3390/ijms252011040. Int J Mol Sci. 2024. PMID: 39456822 Free PMC article. Review.
-
An Integrated In Silico and In Vitro Approach for the Identification of Natural Products Active against SARS-CoV-2.Biomolecules. 2023 Dec 28;14(1):43. doi: 10.3390/biom14010043. Biomolecules. 2023. PMID: 38254643 Free PMC article.
-
Targeting Spike Glycoprotein S1 Mediated by NLRP3 Inflammasome Machinery and the Cytokine Releases in A549 Lung Epithelial Cells by Nanocurcumin.Pharmaceuticals (Basel). 2023 Jun 9;16(6):862. doi: 10.3390/ph16060862. Pharmaceuticals (Basel). 2023. PMID: 37375809 Free PMC article.
-
Luteolin-Rich Extract from Harrisonia perforata (Blanco) Merr. Root Alleviates SARS-CoV-2 Spike Protein-Stimulated Lung Inflammation via Inhibition of MAPK/NLRP3 Inflammasome Signaling Pathways.Life (Basel). 2025 Jul 5;15(7):1077. doi: 10.3390/life15071077. Life (Basel). 2025. PMID: 40724579 Free PMC article.
References
-
- World Health Organization [WHO]. COVID-19 Weekly Epidemiological Update, Edition 110, 21 September 2022. Geneva: WHO; (2022).
-
- Dennis A, Wamil M, Kapur S, Alberts J, Badley A, Decker G, et al. Multi-organ impairment in low-risk individuals with long COVID. medRxiv. [Peprint]. (2020). 10.1101/2020.10.14.20212555 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

