Case report: Sotrovimab, remdesivir and nirmatrelvir/ritonavir combination as salvage treatment option in two immunocompromised patients hospitalized for COVID-19
- PMID: 36698815
- PMCID: PMC9868302
- DOI: 10.3389/fmed.2022.1062450
Case report: Sotrovimab, remdesivir and nirmatrelvir/ritonavir combination as salvage treatment option in two immunocompromised patients hospitalized for COVID-19
Abstract
COVID-19 in immunocompromised patients is difficult to treat. SARS-CoV-2 interaction with the host immune system and the role of therapy still remains only partly understood. There are no data regarding the use of monoclonal antibodies and the combination of two antivirals in fighting viral replication and disease progression. We report the cases of two patients, both treated with rituximab for non-Hodgkin lymphoma and granulomatosis with polyangiitis, respectively, and both hospitalized for COVID-19 with positive SARS-CoV-2 RNAemia, who were successfully treated with a salvage combination therapy with sotrovimab, remdesivir and nirmatrelvir/ritonavir.
Keywords: COVID-19; immunocompromised; monoclonal antibodies; nirmatrelvir/ritonavir; remdesivir; salvage therapy; sotrovimab.
Copyright © 2023 Baldi, Dentone, Mikulska, Fenoglio, Mirabella, Magnè, Portunato, Altosole, Sepulcri, Giacobbe, Uras, Scavone, Taramasso, Orsi, Cittadini, Filaci and Bassetti.
Conflict of interest statement
Outside the submitted work, DG reports investigator-initiated grants from Pfizer, Shionogi, and Gilead Italia and speaker fees and/or advisor fees from Pfizer and Tillotts Pharma. Outside the submitted work, MB reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Bayer, BioMérieux, Cidara, Cipla, Gilead, Menarini, MSD, Pfizer, and Shionogi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
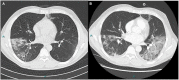
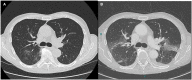

Similar articles
-
Successful treatment of persistent SARS-CoV-2 infection with nirmatrelvir/ritonavir plus sotrovimab in four immunocompromised patients.J Chemother. 2023 Nov;35(7):623-626. doi: 10.1080/1120009X.2023.2196917. Epub 2023 Apr 27. J Chemother. 2023. PMID: 37102326
-
Optimizing COVID-19 treatment in immunocompromised patients: early combination therapy with remdesivir, nirmatrelvir/ritonavir and sotrovimab.Virol J. 2023 Dec 15;20(1):301. doi: 10.1186/s12985-023-02269-8. Virol J. 2023. PMID: 38102675 Free PMC article.
-
Synergistic Activity of Remdesivir-Nirmatrelvir Combination on a SARS-CoV-2 In Vitro Model and a Case Report.Viruses. 2023 Jul 19;15(7):1577. doi: 10.3390/v15071577. Viruses. 2023. PMID: 37515263 Free PMC article.
-
[Early post-exposure and curative therapeutic strategies against COVID-19].Rev Mal Respir. 2024 Jan;41(1):51-58. doi: 10.1016/j.rmr.2023.10.007. Epub 2023 Nov 22. Rev Mal Respir. 2024. PMID: 37993363 Review. French.
-
COVID-19 treatments approved in the European Union and clinical recommendations for the management of non-hospitalized and hospitalized patients.Ann Med. 2022 Dec;54(1):2856-2860. doi: 10.1080/07853890.2022.2133162. Ann Med. 2022. PMID: 36259490 Free PMC article. Review.
Cited by
-
Remdesivir Use in the Real-World Setting: An Overview of Available Evidence.Viruses. 2023 May 14;15(5):1167. doi: 10.3390/v15051167. Viruses. 2023. PMID: 37243253 Free PMC article. Review.
-
Clinical and epidemiological factors causing longer SARS-CoV 2 viral shedding: the results from the CoviCamp cohort.Infection. 2024 Apr;52(2):439-446. doi: 10.1007/s15010-023-02095-8. Epub 2023 Sep 13. Infection. 2024. PMID: 37704910 Free PMC article.
-
Struggling with COVID-19 in Adult Inborn Errors of Immunity Patients: A Case Series of Combination Therapy and Multiple Lines of Therapy for Selected Patients.Life (Basel). 2023 Jul 8;13(7):1530. doi: 10.3390/life13071530. Life (Basel). 2023. PMID: 37511905 Free PMC article.
-
Prolonged infective SARS-CoV-2 omicron variant shedding in a patient with diffuse large B cell lymphoma successfully cleared after three courses of remdesivir.J Infect Chemother. 2023 Aug;29(8):820-824. doi: 10.1016/j.jiac.2023.05.003. Epub 2023 May 12. J Infect Chemother. 2023. PMID: 37182841 Free PMC article.
-
Lessons learnt from broad-spectrum coronavirus antiviral drug discovery.Expert Opin Drug Discov. 2024 Sep;19(9):1023-1041. doi: 10.1080/17460441.2024.2385598. Epub 2024 Jul 30. Expert Opin Drug Discov. 2024. PMID: 39078037 Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

