Rational synthesis, biological screening of azo derivatives of chloro-phenylcarbonyl diazenyl hydroxy dipyrimidines/thioxotetrahydropyrimidines and their metal complexes
- PMID: 36699273
- PMCID: PMC9868376
- DOI: 10.1016/j.heliyon.2022.e12492
Rational synthesis, biological screening of azo derivatives of chloro-phenylcarbonyl diazenyl hydroxy dipyrimidines/thioxotetrahydropyrimidines and their metal complexes
Abstract
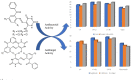
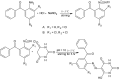
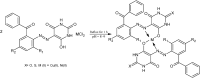
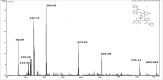
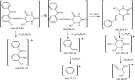
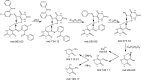
Herein, a new series of azo ligands HL-1 (5-(2-chloro-6-(phenylcarbonyl)phenyl)diazenyl)-6-hydroxydihydropyrimidines-2,4dione), HL-2 (5-(2-chloro-6-(phenylcarbonyl)phenyl)diazenyl)-6-hydroxy-2-thioxottetrahydropyrimidin-4one), HL-3 (5-(2,4-dichloro-6-(phenylcarbonyl)phenyl) diazenyl)-6-hydroxydihydropyrimidines-2,4dione), HL-4 (5-(2,4-dichloro-6-(phenylcarbonyl) phenyl)diazenyl)-6-hydroxy-2-thioxotetrahydropyrimidin-4one) and their metal complexes with Cu(II) & Ni(II) were synthesized successfully having excellent yield, in reproducible conditions and for structure elucidation different advance spectroscopic techniques (FTIR, 1H NMR, 13C NMR and Mass Spectrometry) were applied. In FTIR analysis, the absence of peak at 3450-3550 cm -1 due to -NH2 and presence of a new peak of N=N at 1390-1520 cm-1 confirmed synthesis of the ligands. The 1H NMR spectra of azo ligands showed singlet peak at 11.5-13.5 ppm (Ar-OH) for hydroxyl group and -NH2 signals disappearance of anilines at 4-5 ppm also gives strong indication for the synthesis of azo compounds. On complexation two most important peaks (M-O, M-N) appeared in all the metal chelates in the range of 400-600 cm-1 which were not present in any of the ligands, confirmed the formation of complexes. Molecular ion peaks in mass spectra at 273, 388, 407 and 423 m/z value for ligands as well as for complexes at 803, 835, 871 and 904 m/z also give strong indication that proposed ligands and their metal complexes are produced successfully. Biological screening of the synthesized compounds were also carried out against different bacterial strains (E.coli, S.typhi, and B.subtilis), antifungal (C.albicans, A.niger, and C.glabrata) strains and antioxidant activity. From results it was observed that HL-4 and Cu complexes exhibited maximum inhibition against all bacterial and fungal strains as compared to other ligands and standard drug.
Keywords: Antimicrobial agents; Antioxidant; Azo compounds; Metal complexes.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Spectroscopic studies and biological evaluation of some transition metal complexes of azo Schiff-base ligand derived from (1-phenyl-2,3-dimethyl-4-aminopyrazol-5-one) and 5-((4-chlorophenyl)diazenyl)-2-hydroxybenzaldehyde.Spectrochim Acta A Mol Biomol Spectrosc. 2012 Oct;96:493-500. doi: 10.1016/j.saa.2012.05.053. Epub 2012 Jun 2. Spectrochim Acta A Mol Biomol Spectrosc. 2012. PMID: 22728967
-
Synthesis, Characterization, Antimicrobial and Antioxidant Potential of Diazenyl Chalcones.Curr Top Med Chem. 2018;18(10):844-856. doi: 10.2174/1568026618666180626095714. Curr Top Med Chem. 2018. PMID: 29943703
-
Complex formation, thermal behavior and stability competition between Cu(II) ion and Cu(0) nanoparticles with some new azo dyes. Antioxidant and in vitro cytotoxic activity.Spectrochim Acta A Mol Biomol Spectrosc. 2013 Apr 15;107:359-70. doi: 10.1016/j.saa.2013.01.039. Epub 2013 Jan 31. Spectrochim Acta A Mol Biomol Spectrosc. 2013. PMID: 23434564
-
Metal-Based Antibacterial and Antifungal Agents: Synthesis, Characterization, and In Vitro Biological Evaluation of Co(II), Cu(II), Ni(II), and Zn(II) Complexes With Amino Acid-Derived Compounds.Bioinorg Chem Appl. 2006;2006:83131. doi: 10.1155/BCA/2006/83131. Epub 2006 Dec 20. Bioinorg Chem Appl. 2006. PMID: 17497020 Free PMC article.
-
Synthesis and characterization of Cu(II), Co(II) and Ni(II) complexes of a number of sulfadrug azodyes and their application for wastewater treatment.Spectrochim Acta A Mol Biomol Spectrosc. 2014;121:180-7. doi: 10.1016/j.saa.2013.09.070. Epub 2013 Oct 30. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24239761
Cited by
-
Antibacterial and antioxidant activities of curcumin/Zn metal complex with its chemical characterization and spectroscopic studies.Heliyon. 2023 Jun 21;9(6):e17468. doi: 10.1016/j.heliyon.2023.e17468. eCollection 2023 Jun. Heliyon. 2023. PMID: 37416677 Free PMC article.
-
Silver N-Heterocyclic Carbene (NHC) Complexes as Antimicrobial and/or Anticancer Agents.Pharmaceuticals (Basel). 2024 Dec 25;18(1):9. doi: 10.3390/ph18010009. Pharmaceuticals (Basel). 2024. PMID: 39861072 Free PMC article. Review.
-
Unveiling a novel terpolymer-metal complex: A detailed exploration of synthesis, characterization, and its potential as an antimicrobial and antioxidant agent.Heliyon. 2023 Sep 27;9(10):e20459. doi: 10.1016/j.heliyon.2023.e20459. eCollection 2023 Oct. Heliyon. 2023. PMID: 37810859 Free PMC article.
-
Reaction of quinaldine with 4,6-di(tert-butyl)-3-nitro-1,2-benzoquinone. Dependence of the outcome on the reaction conditions and a deeper insight into the mechanism.Heliyon. 2023 Jun 7;9(6):e16943. doi: 10.1016/j.heliyon.2023.e16943. eCollection 2023 Jun. Heliyon. 2023. PMID: 37389047 Free PMC article.
-
Extraction and characterization of novel alternative cellulosic fiber for sustainable textiles from Aloe barbadensis Miller stems (agricultural waste).Heliyon. 2024 Sep 6;10(18):e37428. doi: 10.1016/j.heliyon.2024.e37428. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309833 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials

