Evaluation of presepsin as a diagnostic tool in newborns with risk of early-onset neonatal sepsis
- PMID: 36699313
- PMCID: PMC9869960
- DOI: 10.3389/fped.2022.1019825
Evaluation of presepsin as a diagnostic tool in newborns with risk of early-onset neonatal sepsis
Abstract
Objectives: To evaluate the efficacy of presepsin (P-SEP) as a potential biomarker of early-onset neonatal sepsis (EOS) and compare it to other routinely used markers of inflammation. To establish the cut-off values of P-SEP for EOS.
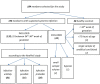
Study design: 184 newborns were prospectively recruited between January 2018 to December 2020. Newborns >34th gestational week with suspected infection were included up to 72 h after delivery, and divided into three categories (i.e., unlikely, possible, and probable infection) based on risk factors, clinical symptoms and laboratory results. Values of plasma P-SEP were sequentially analyzed.
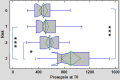
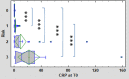
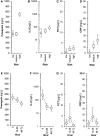
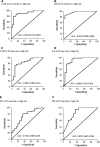
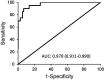
Results: Median values of P-SEP in newborns with probable infection were significantly higher compared to healthy newborns (p = 0.0000013) and unlikely infection group (p = 0.0000025). The AUC for discriminating the probable infection group from the unlikely infection group was 0.845 (95% Cl: 0.708-0.921). The diagnostic efficacy of P-SEP was highest when used in combination with IL-6 and CRP (0.97; 95% CI: 0.911-0.990). The optimal cut-off value of P-SEP was determined to be 695 ng/L.
Conclusion: P-SEP, when combined with IL-6 and CRP, may be utilized as a negative predictive marker of EOS (NPV 97.2%, 95% CI: 93.3-101), especially in newborns at low to medium risk of infection.
Keywords: biomarker; inflammation; neonatal sepsis; newborns; presepsin.
© 2023 Pospisilova, Brodska, Bloomfield, Borecka and Janota.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cord blood presepsin as a predictor of early-onset neonatal sepsis in term and preterm newborns.Ital J Pediatr. 2023 Mar 21;49(1):35. doi: 10.1186/s13052-023-01420-z. Ital J Pediatr. 2023. PMID: 36945009 Free PMC article.
-
Presepsin for the detection of early-onset sepsis in preterm newborns.Pediatr Res. 2017 Feb;81(2):329-334. doi: 10.1038/pr.2016.217. Epub 2016 Nov 3. Pediatr Res. 2017. PMID: 27925621
-
Presepsin as a predictor of positive blood culture in suspected neonatal sepsis.Pediatr Int. 2018 Feb;60(2):157-161. doi: 10.1111/ped.13469. Pediatr Int. 2018. PMID: 29205640
-
Soluble CD14 subtype (sCD14-ST) as biomarker in neonatal early-onset sepsis and late-onset sepsis: a systematic review and meta-analysis.BMC Immunol. 2019 Jun 3;20(1):17. doi: 10.1186/s12865-019-0298-8. BMC Immunol. 2019. PMID: 31159729 Free PMC article.
-
Accuracy of presepsin in neonatal sepsis: systematic review and meta-analysis.Expert Rev Anti Infect Ther. 2019 Apr;17(4):223-232. doi: 10.1080/14787210.2019.1584037. Epub 2019 Mar 8. Expert Rev Anti Infect Ther. 2019. PMID: 30775935
Cited by
-
Can Presepsin Be Valuable in Reducing Unnecessary Antibiotic Exposure after Birth?Antibiotics (Basel). 2023 Apr 2;12(4):695. doi: 10.3390/antibiotics12040695. Antibiotics (Basel). 2023. PMID: 37107057 Free PMC article.
-
Neonatal Infectious Disease: A Major Contributor to Infant Mortality Requiring Advances in Point-of-Care Diagnosis.Antibiotics (Basel). 2024 Sep 13;13(9):877. doi: 10.3390/antibiotics13090877. Antibiotics (Basel). 2024. PMID: 39335050 Free PMC article. Review.
-
Presepsin Levels in Pediatric Patients with Fever and Suspected Sepsis: A Pilot Study in an Emergency Department.Children (Basel). 2024 May 15;11(5):594. doi: 10.3390/children11050594. Children (Basel). 2024. PMID: 38790589 Free PMC article.
-
Biomarkers of Neonatal Sepsis: Where We Are and Where We Are Going.Antibiotics (Basel). 2023 Jul 26;12(8):1233. doi: 10.3390/antibiotics12081233. Antibiotics (Basel). 2023. PMID: 37627653 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

