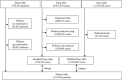
A pharmacovigilance approach for assessing the occurrence of suicide-related events induced by antiepileptic drugs using the Japanese adverse drug event report database
- PMID: 36699485
- PMCID: PMC9868764
- DOI: 10.3389/fpsyt.2022.1091386
A pharmacovigilance approach for assessing the occurrence of suicide-related events induced by antiepileptic drugs using the Japanese adverse drug event report database
Abstract
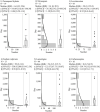
Increased suicidality after antiepileptic drug (AED) treatment remains controversial. This study aimed to investigate the occurrence of suicide-related events (SREs) in Japan. SREs signals with AEDs used orally were evaluated by calculating reporting odds ratios (RORs) and information components (ICs) using the Japanese Adverse Drug Event Report (JADER) database from April 2004 to December 2021. Additionally, factors affecting the occurrence of SREs and time-to-onset from the initial AED treatment were analyzed. Of 22 AEDs, 12 (perampanel hydrate, nitrazepam, levetiracetam, clonazepam, clobazam, sodium valproate, phenobarbital, lamotrigine, lacosamide, gabapentin, zonisamide, and carbamazepine) showed signals of SREs. Patients in their 20 and 30 s, female sex, and concomitant use of multiple AEDs affected the occurrence of SREs. In six AEDs, the median time-to-onset of SREs in patients taking all AEDs was <100 days. The pharmacovigilance approach revealed that several AEDs displayed suicidality signals. Female patients, those in their 20 and 30 s, undergoing combination therapy with ≥2 AEDs, and patients early (<100 days from the initial treatment) in the course of AED therapy should be cautioned about SREs.
Keywords: Japanese adverse drug event report; antiepileptic drugs; perampanel hydrate; pharmacovigilance; suicidality.
Copyright © 2023 Koseki, Horie, Kumazawa, Nakabayashi and Yamada.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Antiepileptic combination therapy with Stevens-Johnson syndrome and toxic epidermal necrolysis: Analysis of a Japanese pharmacovigilance database.Epilepsia. 2020 Sep;61(9):1979-1989. doi: 10.1111/epi.16626. Epub 2020 Aug 6. Epilepsia. 2020. PMID: 32761907
-
Association of Aggression and Antiepileptic Drugs: Analysis Using the Japanese Adverse Drug Event Report (JADER) Database.Biol Pharm Bull. 2022;45(6):720-723. doi: 10.1248/bpb.b21-00954. Biol Pharm Bull. 2022. PMID: 35650100
-
Association of Stevens-Johnson syndrome and toxic epidermal necrolysis with antiepileptic drugs in pediatric patients: Subgroup analysis based on a Japanese spontaneous database.J Clin Pharm Ther. 2019 Oct;44(5):775-779. doi: 10.1111/jcpt.13001. Epub 2019 Jun 23. J Clin Pharm Ther. 2019. PMID: 31231846
-
[Antiepileptic drugs in North America].Brain Nerve. 2010 May;62(5):519-26. Brain Nerve. 2010. PMID: 20450099 Review. Japanese.
-
The problem of osteoporosis in epileptic patients taking antiepileptic drugs.Expert Opin Drug Saf. 2014 Jul;13(7):935-46. doi: 10.1517/14740338.2014.919255. Epub 2014 May 12. Expert Opin Drug Saf. 2014. PMID: 24821596 Review.
Cited by
-
Occurrence of intraocular hemorrhages under monotherapy or combination therapy of antiplatelets and anticoagulants using the Japanese Adverse Drug Event Report database.J Pharm Pharm Sci. 2023 Apr 12;26:11263. doi: 10.3389/jpps.2023.11263. eCollection 2023. J Pharm Pharm Sci. 2023. PMID: 37122387 Free PMC article.
-
Assessing the Labeling Information on Drugs Associated With Suicide Risk: Systematic Review.JMIR Public Health Surveill. 2024 Jan 30;10:e49755. doi: 10.2196/49755. JMIR Public Health Surveill. 2024. PMID: 38289650 Free PMC article.
-
Drug Safety and Suicidality Risk of Chronic Pain Medications.Pharmaceuticals (Basel). 2023 Oct 20;16(10):1497. doi: 10.3390/ph16101497. Pharmaceuticals (Basel). 2023. PMID: 37895968 Free PMC article. Review.
-
Association Between Hypnotics, Accidents, and Injuries: A Study Based on the Adverse Drug Event Reporting Database in Japan.In Vivo. 2025 Jan-Feb;39(1):433-439. doi: 10.21873/invivo.13846. In Vivo. 2025. PMID: 39740920 Free PMC article.
-
Adverse effects of antiseizure medications: a review of the impact of pharmacogenetics and drugs interactions in clinical practice.Front Pharmacol. 2025 Jul 10;16:1584566. doi: 10.3389/fphar.2025.1584566. eCollection 2025. Front Pharmacol. 2025. PMID: 40709084 Free PMC article. Review.
References
-
- FDA. Statistical Review and Evaluation: Antiepileptic Drugs and Suicidality. Silver Spring, MD: FDA; (2008).
LinkOut - more resources
Full Text Sources
Miscellaneous