Hypothalamic-pituitary-adrenal axis and renin-angiotensin-aldosterone system in adulthood PTSD and childhood maltreatment history
- PMID: 36699501
- PMCID: PMC9869036
- DOI: 10.3389/fpsyt.2022.967779
Hypothalamic-pituitary-adrenal axis and renin-angiotensin-aldosterone system in adulthood PTSD and childhood maltreatment history
Abstract
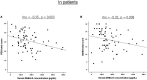
Accumulated evidence shows that psychological trauma and posttraumatic stress disorder (PTSD) are associated with dysfunction in the hypothalamic-pituitary-adrenal (HPA) axis. Besides the HPA axis hormones, recent evidence suggests that the renin-angiotensin-aldosterone (RAA) system and genetic factors may be involved in trauma/PTSD as well as in HPA axis regulation. This study attempted to better understand the HPA axis function in relation to PTSD and childhood maltreatment by simultaneously examining RAA system and genetic polymorphisms of candidate genes. Here we studied 69 civilian women with PTSD and 107 healthy control women without DSM-IV-based traumatic experience. Childhood maltreatment history was assessed with the Childhood Trauma Questionnaire. PTSD severity was assessed with the Posttraumatic Diagnostic Scale. Functional disability was assessed with the Sheehan Disability Scale. HPA axis was examined by measuring blood levels of cortisol, adrenocorticotropic hormone, and dehydroepiandrosterone-sulphate (DHEA-S). RAA system was examined by measuring blood renin and aldosterone levels. The FKBP5 rs1360780 and CACNA1C rs1006737 polymorphisms were genotyped. No significant differences were seen between patients and controls in any of the five hormone levels. DHEA-S levels were significantly negatively correlated with overall PTSD severity (p = 0.003) and functional disability (p = 0.008). A two-way analysis of variance with diagnostic groups and genotypes as fixed factors revealed that patients with the rs1006737 A-allele had significantly lower DHEA-S levels than patients with the GG genotype (p = 0.002) and controls with the A-allele (p = 0.006). Childhood maltreatment history was not significantly correlated with any of the five hormone levels. These results were generally unchanged after controlling for the potentially confounding effect of age, depression, and anxiety. Our findings suggest that lower DHEA-S levels could indicate more severe subtype of PTSD, the association of which might be partly modified by the CACNA1C polymorphism.
Keywords: childhood maltreatment; dehydroepiandrosterone-sulphate (DHEA-S); gene; hypothalamic-pituitary-adrenal (HPA) axis; polymorphism; posttraumatic stress disorder (PTSD); renin-angiotensin-aldosterone (RAA) system.
Copyright © 2023 Kakehi, Hori, Yoshida, Itoh, Lin, Niwa, Narita, Ino, Imai, Sasayama, Kamo, Kunugi and Kim.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The associations of hair cortisol and DHEA with posttraumatic stress disorder symptoms in refugees.Compr Psychiatry. 2024 Feb;129:152438. doi: 10.1016/j.comppsych.2023.152438. Epub 2023 Nov 22. Compr Psychiatry. 2024. PMID: 38104462
-
Correlation between hypothalamic-pituitary-adrenal axis gene polymorphisms and posttraumatic stress disorder symptoms.Horm Behav. 2020 Jan;117:104604. doi: 10.1016/j.yhbeh.2019.104604. Epub 2019 Nov 6. Horm Behav. 2020. PMID: 31655035
-
The relationship between childhood trauma, rs1360780 genotypes, FKBP5 intron 7 methylation and posttraumatic stress disorder in women who have experienced rape.Eur J Psychotraumatol. 2025 Dec;16(1):2485707. doi: 10.1080/20008066.2025.2485707. Epub 2025 Apr 17. Eur J Psychotraumatol. 2025. PMID: 40242984 Free PMC article.
-
DHEA and DHEA-S levels in posttraumatic stress disorder: A meta-analytic review.Psychoneuroendocrinology. 2017 Oct;84:76-82. doi: 10.1016/j.psyneuen.2017.06.010. Epub 2017 Jun 15. Psychoneuroendocrinology. 2017. PMID: 28668711 Review.
-
Implications of hypothalamic-pituitary-adrenal axis functioning in posttraumatic stress disorder.J Am Psychiatr Nurses Assoc. 2011 Nov-Dec;17(6):393-403. doi: 10.1177/1078390311420564. J Am Psychiatr Nurses Assoc. 2011. PMID: 22142976 Review.
Cited by
-
Genetic, Epigenetic, and Hormonal Regulation of Stress Phenotypes in Major Depressive Disorder: From Maladaptation to Resilience.Cell Mol Neurobiol. 2025 Mar 26;45(1):29. doi: 10.1007/s10571-025-01549-x. Cell Mol Neurobiol. 2025. PMID: 40138049 Free PMC article. Review.
-
The Effects of Bioactive Compounds on PTSD Treatment.Curr Neuropharmacol. 2025;23(10):1156-1168. doi: 10.2174/011570159X333438240927103741. Curr Neuropharmacol. 2025. PMID: 39354772 Free PMC article. Review.
-
Current Status and Future Directions of Artificial Intelligence in Post-Traumatic Stress Disorder: A Literature Measurement Analysis.Behav Sci (Basel). 2024 Dec 30;15(1):27. doi: 10.3390/bs15010027. Behav Sci (Basel). 2024. PMID: 39851830 Free PMC article. Review.
-
Efficacy of spironolactone as adjunctive therapy to sodium valproate in bipolar-I disorder: A double-blind, randomized, placebo-controlled clinical trial.Brain Behav. 2023 Dec;13(12):e3313. doi: 10.1002/brb3.3313. Epub 2023 Nov 6. Brain Behav. 2023. PMID: 37933420 Free PMC article. Clinical Trial.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

