Piecewise quadratic neuron model: A tool for close-to-biology spiking neuronal network simulation on dedicated hardware
- PMID: 36699524
- PMCID: PMC9870328
- DOI: 10.3389/fnins.2022.1069133
Piecewise quadratic neuron model: A tool for close-to-biology spiking neuronal network simulation on dedicated hardware
Abstract
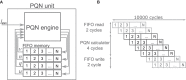
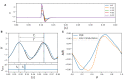
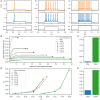
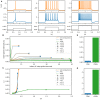
Spiking neuron models simulate neuronal activities and allow us to analyze and reproduce the information processing of the nervous system. However, ionic-conductance models, which can faithfully reproduce neuronal activities, require a huge computational cost, while integral-firing models, which are computationally inexpensive, have some difficulties in reproducing neuronal activities. Here we propose a Piecewise Quadratic Neuron (PQN) model based on a qualitative modeling approach that aims to reproduce only the key dynamics behind neuronal activities. We demonstrate that PQN models can accurately reproduce the responses of ionic-conductance models of major neuronal classes to stimulus inputs of various magnitudes. In addition, the PQN model is designed to support the efficient implementation on digital arithmetic circuits for use as silicon neurons, and we confirm that the PQN model consumes much fewer circuit resources than the ionic-conductance models. This model intends to serve as a tool for building a large-scale closer-to-biology spiking neural network.
Keywords: FPGA; PQN model; silicon neuron; silicon neuronal network; spiking neuron model.
Copyright © 2023 Nanami and Kohno.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








Similar articles
-
A lightweight data-driven spiking neuronal network model of Drosophila olfactory nervous system with dedicated hardware support.Front Neurosci. 2024 Jun 26;18:1384336. doi: 10.3389/fnins.2024.1384336. eCollection 2024. Front Neurosci. 2024. PMID: 38994271 Free PMC article.
-
Simple Cortical and Thalamic Neuron Models for Digital Arithmetic Circuit Implementation.Front Neurosci. 2016 May 13;10:181. doi: 10.3389/fnins.2016.00181. eCollection 2016. Front Neurosci. 2016. PMID: 27242397 Free PMC article.
-
Qualitative-Modeling-Based Silicon Neurons and Their Networks.Front Neurosci. 2016 Jun 15;10:273. doi: 10.3389/fnins.2016.00273. eCollection 2016. Front Neurosci. 2016. PMID: 27378842 Free PMC article. Review.
-
FPGA implementation of a complete digital spiking silicon neuron for circuit design and network approach.Sci Rep. 2025 Mar 12;15(1):8491. doi: 10.1038/s41598-025-92570-z. Sci Rep. 2025. PMID: 40075086 Free PMC article.
-
Neuromorphic hardware databases for exploring structure-function relationships in the brain.Philos Trans R Soc Lond B Biol Sci. 2001 Aug 29;356(1412):1249-58. doi: 10.1098/rstb.2001.0904. Philos Trans R Soc Lond B Biol Sci. 2001. PMID: 11545701 Free PMC article. Review.
Cited by
-
A lightweight data-driven spiking neuronal network model of Drosophila olfactory nervous system with dedicated hardware support.Front Neurosci. 2024 Jun 26;18:1384336. doi: 10.3389/fnins.2024.1384336. eCollection 2024. Front Neurosci. 2024. PMID: 38994271 Free PMC article.
References
LinkOut - more resources
Full Text Sources

