Bone Formation in 2D Culture of Primary Cells
- PMID: 36699640
- PMCID: PMC9850442
- DOI: 10.1002/jbm4.10701
Bone Formation in 2D Culture of Primary Cells
Abstract
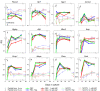
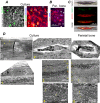
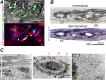
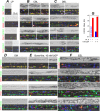
Relevance of mineralized nodules in two-dimensional (2D) osteoblast/osteocyte cultures to bone biology, pathology, and engineering is a decades old question, but a comprehensive answer appears to be still wanting. Bone-like cells, extracellular matrix (ECM), and mineral were all reported but so were non-bone-like ones. Many studies described seemingly bone-like cell-ECM structures based on similarity to few select bone features in vivo, yet no studies examined multiple bone features simultaneously and none systematically studied all types of structures coexisting in the same culture. Here, we report such comprehensive analysis of 2D cultures based on light and electron microscopies, Raman microspectroscopy, gene expression, and in situ messenger RNA (mRNA) hybridization. We demonstrate that 2D cultures of primary cells from mouse calvaria do form bona fide bone. Cells, ECM, and mineral within it exhibit morphology, structure, ultrastructure, composition, spatial-temporal gene expression pattern, and growth consistent with intramembranous ossification. However, this bone is just one of at least five different types of cell-ECM structures coexisting in the same 2D culture, which vary widely in their resemblance to bone and ability to mineralize. We show that the other two mineralizing structures may represent abnormal (disrupted) bone and cartilage-like structure with chondrocyte-to-osteoblast transdifferentiation. The two nonmineralizing cell-ECM structures may mimic periosteal cambium and pathological, nonmineralizing osteoid. Importantly, the most commonly used culture conditions (10mM β-glycerophosphate) induce artificial mineralization of all cell-ECM structures, which then become barely distinguishable. We therefore discuss conditions and approaches promoting formation of bona fide bone and simple means for distinguishing it from the other cell-ECM structures. Our findings may improve osteoblast differentiation and function analyses based on 2D cultures and extend applications of these cultures to general bone biology and tissue engineering research. Published 2022. This article is a U.S. Government work and is in the public domain in the USA. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Keywords: CHONDROCYTES; COLLAGEN; MATRIX MINERALIZATION; MC3T3; OSTEOBLASTS; OSTEOCYTES.
Published 2022. This article is a U.S. Government work and is in the public domain in the USA. JBMR Plus published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research.
Conflict of interest statement
All authors declare having no conflicts of interest and not being subjects of any institutional investigations.
Figures






Similar articles
-
Type VI Collagen Regulates Endochondral Ossification in the Temporomandibular Joint.JBMR Plus. 2022 Mar 10;6(5):e10617. doi: 10.1002/jbm4.10617. eCollection 2022 May. JBMR Plus. 2022. PMID: 35509631 Free PMC article.
-
Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: an ultrastructural, compositional and comparative analysis with mouse bone.Bone. 2015 Feb;71:244-56. doi: 10.1016/j.bone.2014.11.003. Epub 2014 Nov 13. Bone. 2015. PMID: 25460184 Free PMC article.
-
Osteocyte-Like Cells Differentiated From Primary Osteoblasts in an Artificial Human Bone Tissue Model.JBMR Plus. 2023 Jun 28;7(9):e10792. doi: 10.1002/jbm4.10792. eCollection 2023 Sep. JBMR Plus. 2023. PMID: 37701151 Free PMC article.
-
Extracellular matrix networks in bone remodeling.Int J Biochem Cell Biol. 2015 Aug;65:20-31. doi: 10.1016/j.biocel.2015.05.008. Epub 2015 May 18. Int J Biochem Cell Biol. 2015. PMID: 25997875 Review.
-
Matrix Vesicle-Mediated Mineralization and Osteocytic Regulation of Bone Mineralization.Int J Mol Sci. 2022 Sep 1;23(17):9941. doi: 10.3390/ijms23179941. Int J Mol Sci. 2022. PMID: 36077336 Free PMC article. Review.
Cited by
-
Osteoclast-derived coupling factors: origins and state-of-play Louis V Avioli lecture, ASBMR 2023.J Bone Miner Res. 2024 Sep 26;39(10):1377-1385. doi: 10.1093/jbmr/zjae110. J Bone Miner Res. 2024. PMID: 38990205 Free PMC article.
-
Long-Term Culture of Cellular Spheroids in Novel Hydrogel Constructs for ECM Characterization in Bone Models.Materials (Basel). 2025 Jul 28;18(15):3538. doi: 10.3390/ma18153538. Materials (Basel). 2025. PMID: 40805416 Free PMC article.
-
RNA-based bone histomorphometry: method and its application to explaining postpubertal bone gain in a G610C mouse model of osteogenesis imperfecta.J Bone Miner Res. 2024 Mar 22;39(2):177-189. doi: 10.1093/jbmr/zjad004. J Bone Miner Res. 2024. PMID: 38477760 Free PMC article.
References
-
- Bilezikian JP, ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. 9th ed. Wiley‐Blackwell: Hoboken, NJ; 2019.
-
- Bronner F, Farach‐Carson MC, Roach HI, eds. Bone and Development. London: Springer; 2010.
-
- Thakker RV, Whyte MP, Eisman JA, Igarashi T, eds. Genetics of Bone Biology and Skeletal Disease. 2nd ed. Academic Press; 2017.
LinkOut - more resources
Full Text Sources
