Induction of 2n pollen with colchicine during microsporogenesis in Phalaenopsis
- PMID: 36699823
- PMCID: PMC9868330
- DOI: 10.1270/jsbbs.21100
Induction of 2n pollen with colchicine during microsporogenesis in Phalaenopsis
Abstract
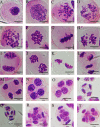
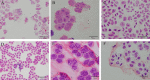
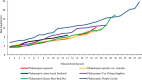
The induction of 2n pollen is an important technique for breeding polyploid plants. Here, we observed meiosis in the pollen mother cells (PMCs) of six Phalaenopsis cultivars and attempted to induce 2n pollen. The meiotic stage was related to flower bud length. During meiosis, Phalaenopsis cultivars with flower widths of approximately 20-40 mm and 50-60 mm had bud lengths of approximately 3-8 mm and 5-13 mm, respectively. The duration of meiosis ranged from 4.2 to 14 d. This was the first study to characterize meiosis of the PMCs of Phalaenopsis. The natural generation frequency of 2n pollen varied from 0.68% to 1.78%. Meiotic stage and colchicine concentration significantly affected the induction of 2n pollen. The most effective treatment for obtaining 2n pollen was 0.05% colchicine in the leptotene to zygotene stage for 3 d, which achieved a 2n pollen frequency of 10.04%.
Keywords: 2n pollen; colchicine; flower bud growth; meiosis stage.
Copyright © 2022 by JAPANESE SOCIETY OF BREEDING.
Figures





Similar articles
-
Effects of Colchicine on Populus canescens Ectexine Structure and 2n Pollen Production.Front Plant Sci. 2020 Mar 17;11:295. doi: 10.3389/fpls.2020.00295. eCollection 2020. Front Plant Sci. 2020. PMID: 32256514 Free PMC article.
-
Colchicine induction of 'Old Blush' 2n pollen for the hybridization and breeding of tetraploid rose.PeerJ. 2021 Mar 9;9:e11043. doi: 10.7717/peerj.11043. eCollection 2021. PeerJ. 2021. PMID: 33854842 Free PMC article.
-
Cytological and morphology characteristics of natural microsporogenesis within Camellia oleifera.Physiol Mol Biol Plants. 2021 May;27(5):959-968. doi: 10.1007/s12298-021-01002-5. Epub 2021 May 16. Physiol Mol Biol Plants. 2021. PMID: 34092947 Free PMC article.
-
Exploitation of induced 2n-gametes for plant breeding.Plant Cell Rep. 2014 Feb;33(2):215-23. doi: 10.1007/s00299-013-1534-y. Epub 2013 Dec 6. Plant Cell Rep. 2014. PMID: 24311154 Review.
-
The time and duration of meiosis.Philos Trans R Soc Lond B Biol Sci. 1977 Mar 21;277(955):201-26. doi: 10.1098/rstb.1977.0012. Philos Trans R Soc Lond B Biol Sci. 1977. PMID: 16285 Review.
Cited by
-
Breeding of ornamental orchids with focus on Phalaenopsis: current approaches, tools, and challenges for this century.Heredity (Edinb). 2024 Apr;132(4):163-178. doi: 10.1038/s41437-024-00671-8. Epub 2024 Feb 2. Heredity (Edinb). 2024. PMID: 38302667 Free PMC article. Review.
-
The Breeding, Cultivation, and Potential Applications of Ornamental Orchids with a Focus on Phalaenopsis-A Brief Review.Plants (Basel). 2025 May 31;14(11):1689. doi: 10.3390/plants14111689. Plants (Basel). 2025. PMID: 40508363 Free PMC article. Review.
References
-
- Ahmed, D., Evrard J.C., Ollitrault P. and Froelicher Y. (2020) The effect of cross direction and ploidy level on phenotypic variation of reciprocal diploid and triploid mandarin hybrids. Tree Genet Genomes 16: 1–16.
-
- Aleza, P., Juárez J., Cuenca J., Ollitrault P. and Navarro L. (2012) Extensive citrus triploid hybrid production by 2x × 4x sexual hybridizations and parent-effect on the length of the juvenile phase. Plant Cell Rep 31: 1723–1735. - PubMed
-
- Andrada, A.R., Ángeles P.V. and Bulacio E. (2019) Chromosome numbers and meiotic analysis in three species of Tropaeolum (Tropaeolaceae). Gayana Bot 76: 84–90.
-
- Azmi, T.K.K., Sukma D., Aziz S.A. and Syukur M. (2016) Morphology and growth of plantlets resulted from polyploidy induction by colchicine treatment on flower buds of moth orchid (Phalaenopsis amabilis (L.) Blume). Jurnal Agronomi Indonesia 44: 68–75 (in Indonesian with English summary).
-
- Baduel, P., Bray S., Vallejo-Marin M., Kolář F. and Yant L. (2018) The “Polyploid Hop”: Shifting challenges and opportunities over the evolutionary lifespan of genome duplications. Front Ecol Evol 6: 117.

