Effect of nasal irrigation in adults infected with Omicron variant of COVID-19: A quasi-experimental study
- PMID: 36699894
- PMCID: PMC9868717
- DOI: 10.3389/fpubh.2022.1046112
Effect of nasal irrigation in adults infected with Omicron variant of COVID-19: A quasi-experimental study
Abstract
Objective: To investigate the effect of nasal irrigation on the duration of symptoms and nucleic acid conversion in adults infected with the Omicron variant of COVID-19.
Methods: This quasi-experimental study enrolled patients diagnosed with asymptomatic, mild, or moderate Omicron infection at the Shandong Public Health Clinical Center between April 1, 2022 and May 1, 2022. Patients were divided into two groups to receive Lianhua Qingwen granules and traditional Chinese medicine (TCM) prescriptions (conventional group) and 3% hypertonic saline nasal irrigation based on conventional treatment (nasal irrigation groups), respectively. Primary outcomes were symptom disappearance time and nucleic acid negative conversion time. Secondary outcomes were peripheral blood white blood cell (WBC), lymphocyte (LYM) count, neutrophil (NEU) count, C-reactive protein (CRP) level, and chest CT examination findings.
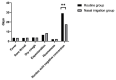
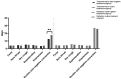
Results: Eighty patients were included (40 patients/group). Multiple linear regression analysis showed that, after adjustment for comorbidities, smoking history, LYM count, and Ct values of N gene, the patients in the nasal irrigation group were more likely to get lower nucleic acid negative conversion time (β = -11.052, 95% CI: -8.277-13.827, P < 0.001) compared with the conventional group. The symptom disappearance time showed no significant improvement (P > 0.05). Subgroup analysis for treatment-naïve patients in the nasal irrigation group showed similar nucleic acid negative conversion time improvement (P = 0.038).
Conclusion: Early nasal irrigation shortens the nucleic acid negative conversion time in adults infected with the Omicron variant but without improvements in symptom disappearance time.
Keywords: COVID-19; Omicron; clinical study; nasal irrigation; quasi-experimental study.
Copyright © 2023 Liu, Xie, Li, Su and Zhu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Effect of Nasal Irrigation in Children With Omicron Variant of COVID-19 Infection.Ear Nose Throat J. 2024 Jun;103(1_suppl):54S-59S. doi: 10.1177/01455613231172337. Epub 2023 Jun 17. Ear Nose Throat J. 2024. PMID: 37329222 Free PMC article.
-
[Characteristics and related factors of viral nucleic acid negative conversion in children infected with Omicron variant strain of SARS-CoV-2].Zhonghua Er Ke Za Zhi. 2022 Dec 2;60(12):1307-1311. doi: 10.3760/cma.j.cn112140-20220623-00582. Zhonghua Er Ke Za Zhi. 2022. PMID: 36444435 Chinese.
-
Efficacy and Safety of Huashi Baidu Granules in Treating Patients with SARS-CoV-2 Omicron Variant: A Single-Center Retrospective Cohort Study.Chin J Integr Med. 2024 Feb;30(2):107-114. doi: 10.1007/s11655-023-3549-8. Epub 2023 May 24. Chin J Integr Med. 2024. PMID: 37222827 Free PMC article.
-
Do saline water gargling and nasal irrigation confer protection against COVID-19?Explore (NY). 2021 Mar-Apr;17(2):127-129. doi: 10.1016/j.explore.2020.09.010. Epub 2020 Oct 1. Explore (NY). 2021. PMID: 33046408 Free PMC article. Review.
-
Efficacy of nasal irrigation with hypertonic saline on chronic rhinosinusitis: systematic review and meta-analysis.Braz J Otorhinolaryngol. 2020 Sep-Oct;86(5):639-646. doi: 10.1016/j.bjorl.2020.03.008. Epub 2020 May 16. Braz J Otorhinolaryngol. 2020. PMID: 32534983 Free PMC article.
Cited by
-
SARS-CoV-2 mechanisms of cell tropism in various organs considering host factors.Heliyon. 2024 Feb 20;10(4):e26577. doi: 10.1016/j.heliyon.2024.e26577. eCollection 2024 Feb 29. Heliyon. 2024. PMID: 38420467 Free PMC article. Review.
-
A Hypertonic Seawater Nasal Irrigation Solution Containing Algal and Herbal Natural Ingredients Reduces Viral Load and SARS-CoV-2 Detection Time in the Nasal Cavity.J Pers Med. 2023 Jul 3;13(7):1093. doi: 10.3390/jpm13071093. J Pers Med. 2023. PMID: 37511706 Free PMC article.
-
Washing Illness Away: A Systematic Review of the Impact of Nasal Irrigation and Spray on COVID-19.Laryngoscope. 2025 Feb;135(2):517-528. doi: 10.1002/lary.31761. Epub 2024 Sep 13. Laryngoscope. 2025. PMID: 39268910 Free PMC article.
-
Estimating the association between cigarette and e-cigarette use patterns and SARS-CoV-2 negative conversion time: retrospective online survey in China.BMC Infect Dis. 2025 Feb 3;25(1):161. doi: 10.1186/s12879-025-10545-x. BMC Infect Dis. 2025. PMID: 39901130 Free PMC article.
-
Significance of saline nasalirrigation for COVID-19 infection: observations and reflections from nursing care of nasopharyngeal carcinoma.Transl Cancer Res. 2024 Feb 29;13(2):1114-1124. doi: 10.21037/tcr-23-2384. Epub 2024 Feb 28. Transl Cancer Res. 2024. PMID: 38482412 Free PMC article.
References
-
- World Health Organization . Coronavirus Disease 2019 (COVID-19). Situation Report−51. March 11, 2020. World Health Organization (2020). Available online at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/2... (accessed December 2, 2022).
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

