Obinutuzumab Effectively Depletes Key B-cell Subsets in Blood and Tissue in End-stage Renal Disease Patients
- PMID: 36700064
- PMCID: PMC9851678
- DOI: 10.1097/TXD.0000000000001436
Obinutuzumab Effectively Depletes Key B-cell Subsets in Blood and Tissue in End-stage Renal Disease Patients
Abstract
The THEORY study evaluated the effects of single and multiple doses of obinutuzumab, a type 2 anti-CD20 antibody that induces antibody-dependent cell-mediated cytotoxicity and direct cell death, in combination with standard of care in patients with end-stage renal disease.
Methods: We measured B-cell subsets and protein biomarkers of B-cell activity in peripheral blood before and after obinutuzumab administration in THEORY patients, and B-cell subsets in lymph nodes in THEORY patients and an untreated comparator cohort.
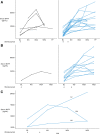
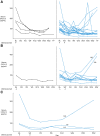
Results: Obinutuzumab treatment resulted in a rapid loss of B-cell subsets (including naive B, memory B, double-negative, immunoglobulin D+ transitional cells, and plasmablasts/plasma cells) in peripheral blood and tissue. This loss of B cells was associated with increased B cell-activating factor and decreased CXCL13 levels in circulation.
Conclusions: Our data further characterize the mechanistic profile of obinutuzumab and suggest that it may elicit greater efficacy in indications such as lupus where B-cell targeting therapeutics are limited by the resistance of pathogenic tissue B cells to depletion.
Copyright © 2023 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
C.M.L., T.S., and S.M. are/were employees of F. Hoffmann-La Roche and own Roche stock and/or options. A.S., J.G., J.K., J.E., C.D.A., and A.M. are/were employees of Genentech, Inc., a member of the Roche group, and own Roche stock and/or options. R.R.R. served on the advisory board for this study. S.C.J. received grants and consultation fees from Hansa Biopharma, Regeneron, Vera, CareDx, and Argenx; and grants, stock options, and IP from CSL-Behring. S.B. is a consultant for Genentech and Gigagen. The other author declare no conflicts of interest.
Figures



References
-
- Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–3908. - PubMed
-
- Rovin BH, Furie R, Latinis K, et al. ; LUNAR Investigator Group. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum. 2012;64:1215–1226. - PubMed
-
- Hawker K, O’Connor P, Freedman MS, et al. ; OLYMPUS trial group. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66:460–471. - PubMed
LinkOut - more resources
Full Text Sources

