SARS-CoV-2 laboratory surveillance during the first year of the COVID-19 pandemic in southern Brazil
- PMID: 36700597
- PMCID: PMC9870280
- DOI: 10.1590/0037-8682-0146-2022
SARS-CoV-2 laboratory surveillance during the first year of the COVID-19 pandemic in southern Brazil
Abstract
Background: Brazil has one of the highest numbers of COVID-19 cases and deaths. Rio Grande do Sul (RS) in southern Brazil is one of the leading states in terms of case numbers. As part of the national public health network, the State Central Laboratory (LACEN-RS) changed its routine in 2020 to focus on the diagnosis of COVID-19. This study evaluated the laboratory surveillance of COVID-19 suspected cases analyzed at the LACEN-RS in 2020.
Methods: Viral detection was performed using RT-qPCR in samples from patients with respiratory infection who met the study criteria. Viral RNA was isolated using commercial manual kits or automated extractors, and SARS-CoV-2 RT-qPCR was performed using the Bio-Manguinhos/Rio de Janeiro, IBMP/Paraná, or Allplex 2019-nCoV assay. In total, 360 representative SARS-CoV-2 samples were sequenced using the Illumina platform.
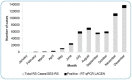
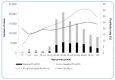
Results: In total, 31,197 of 107,578 (positivity rate = 29%) tested positive for SARS-CoV-2. The number of RT-qPCR tests performed per month followed the COVID-19 epidemic curve observed for the state, with peaks in July-August and December. Females accounted for 63% of the samples, whereas the positivity rate was higher among males (33.1% males vs. 26.5% females). The positivity rate was higher in adults aged 50-79 years compared to the overall positivity rate. The majority of cases were observed in the capital, Porto Alegre, and the metropolitan region. Ten distinct lineages were identified, with B.1.1.28, B.1.1.33, and P.2 being the most frequent.
Conclusions: Here, we describe laboratory surveillance of COVID-19 to identify priorities for epidemiological surveillance actions in RS.
Conflict of interest statement
Figures


Similar articles
-
Influenza outbreak during the surge of SARS-CoV-2 omicron in a metropolitan area from southern Brazil: genomic surveillance.J Med Virol. 2024 Oct;96(10):e29944. doi: 10.1002/jmv.29944. J Med Virol. 2024. PMID: 39360646
-
SARS-CoV-2 introduction and lineage dynamics across three epidemic peaks in Southern Brazil: massive spread of P.1.Infect Genet Evol. 2021 Dec;96:105144. doi: 10.1016/j.meegid.2021.105144. Epub 2021 Nov 17. Infect Genet Evol. 2021. PMID: 34798321 Free PMC article.
-
Environmental monitoring of SARS-CoV-2 in the metropolitan area of Porto Alegre, Rio Grande do Sul (RS), Brazil.Environ Sci Pollut Res Int. 2024 Jan;31(2):2129-2144. doi: 10.1007/s11356-023-31081-8. Epub 2023 Dec 6. Environ Sci Pollut Res Int. 2024. PMID: 38057673 Free PMC article.
-
Antibody tests for identification of current and past infection with SARS-CoV-2.Cochrane Database Syst Rev. 2020 Jun 25;6(6):CD013652. doi: 10.1002/14651858.CD013652. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD013652. doi: 10.1002/14651858.CD013652.pub2. PMID: 32584464 Free PMC article. Updated.
-
Health surveillance in tackling COVID-19 in Brazil: a scope review.Cien Saude Colet. 2025 May;30(5):e02202025. doi: 10.1590/1413-81232025305.02202025. Epub 2025 Feb 12. Cien Saude Colet. 2025. PMID: 40465915 English, Portuguese.
References
-
- World Health Organization (WHO) COVID-19 - China. Off Ctry City, Wuhan Prov Hubei Seafood, Huanan Munic Wuhan Comm Heal. 2020. pp. 1–3.https://www.who.int/docs/default-source/coronaviruse/situation-reports/2...
-
- World Health Organization (WHO) WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February. WHO Dir Gen Statement; 2020. https://www.who.int/director-general/speeches/detail/who-director-genera...
-
- World Health Organization (WHO) Naming the coronavirus disease (COVID-19) and the virus that causes it. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technica...
-
- World Health Organization (WHO) Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases. WHO - Interim Guid; [2020]. 2019. pp. 1–7.
-
- Johns Hopkins University and Medicine Coronavirus resource center. 2021. [2021 Aug 24]. Available from: https://coronavirus.jhu.edu/map.html .
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

