On the Behavior of the Ethylene Glycol Components of Polydisperse Polyethylene Glycol PEG200
- PMID: 36700884
- PMCID: PMC9923754
- DOI: 10.1021/acs.jpcb.2c06773
On the Behavior of the Ethylene Glycol Components of Polydisperse Polyethylene Glycol PEG200
Abstract
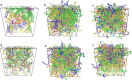
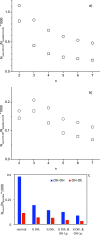
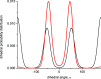
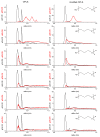
Molecular dynamics (MD) simulations are reported for [polyethylene glycol (PEG)200], a polydisperse mixture of ethylene glycol oligomers with an average molar weight of 200 g·mol-1. As a first step, available force fields for describing ethylene glycol oligomers were tested on how accurately they reproduced experimental properties. They were found to all fall short on either reproducing density, a static property, or the self-diffusion coefficient, a dynamic property. Discrepancies with the experimental data increased with the increasing size of the tested ethylene glycol oligomer. From the available force fields, the optimized potential for liquid simulation (OPLS) force field was used to further investigate which adjustments to the force field would improve the agreement of simulated physical properties with experimental ones. Two parameters were identified and adjusted, the (HO)-C-C-O proper dihedral potential and the polarity of the hydroxy group. The parameter adjustments depended on the size of the ethylene glycol oligomer. Next, PEG200 was simulated with the OPLS force field with and without modifications to inspect their effects on the simulation results. The modifications to the OPLS force field significantly decreased hydrogen bonding overall and increased the propensity of intramolecular hydrogen bond formation at the cost of intermolecular hydrogen bond formation. Moreover, some of the tri- and more so tetraethylene glycol formed intramolecular hydrogen bonds between the hydroxy end groups while still maintaining strong intramolecular interactions with the ether oxygen atoms. These observations allowed the interpretation of the obtained RDFs as well as structural properties such as the average end-to-end distances and the average radii of gyration. The MD simulations with and without the modifications showed no evidence of preferential association of like-oligomers to form clusters nor any evidence of long-range ordering such as a side-by-side stacking of ethylene glycol oligomers. Instead, the simulation results support the picture of PEG200 being a random mixture of its ethylene glycol oligomer components. Finally, additional MD simulations of a binary mixture of tri-and hexaethylene glycol with the same average molar weight as PEG200 revealed very similar structural and physical properties as for PEG200.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Thermophysical properties of polyethylene glycol oligomers via molecular dynamics simulations.RSC Adv. 2024 Sep 3;14(38):28125-28137. doi: 10.1039/d4ra04898a. eCollection 2024 Aug 29. RSC Adv. 2024. PMID: 39228756 Free PMC article.
-
Molecular Dynamics Study of the Green Solvent Polyethylene Glycol with Water Impurities.Molecules. 2024 Apr 30;29(9):2070. doi: 10.3390/molecules29092070. Molecules. 2024. PMID: 38731561 Free PMC article.
-
The Interplay of Inter- and Intramolecular Hydrogen Bonding in Ether Alcohols Related to n-Octanol.Molecules. 2025 Jun 4;30(11):2456. doi: 10.3390/molecules30112456. Molecules. 2025. PMID: 40509343 Free PMC article.
-
Insights Gained from Refined Force-Field for Pure and Aqueous Ethylene Glycol through Molecular Dynamics Simulations.J Phys Chem B. 2019 Aug 1;123(30):6543-6553. doi: 10.1021/acs.jpcb.9b03950. Epub 2019 Jul 23. J Phys Chem B. 2019. PMID: 31335141
-
Intramolecular Hydrogen Bonds in Low-Molecular-Weight Polyethylene Glycol.Chemphyschem. 2016 Apr 18;17(8):1143-53. doi: 10.1002/cphc.201501182. Epub 2016 Feb 19. Chemphyschem. 2016. PMID: 26864943
Cited by
-
ih-RIDME: a pulse EPR experiment to probe the heterogeneous nuclear environment.Magn Reson (Gott). 2025 Mar 10;6(1):93-112. doi: 10.5194/mr-6-93-2025. eCollection 2025. Magn Reson (Gott). 2025. PMID: 40657608 Free PMC article.
-
Thermophysical properties of polyethylene glycol oligomers via molecular dynamics simulations.RSC Adv. 2024 Sep 3;14(38):28125-28137. doi: 10.1039/d4ra04898a. eCollection 2024 Aug 29. RSC Adv. 2024. PMID: 39228756 Free PMC article.
-
NMR and MD Simulations of Non-Ionic Surfactants.Molecules. 2025 Jan 14;30(2):309. doi: 10.3390/molecules30020309. Molecules. 2025. PMID: 39860179 Free PMC article. Review.
-
Structures and Dynamics of Complex Guest Molecules in Confinement, Revealed by Solid-State NMR, Molecular Dynamics, and Calorimetry.Molecules. 2024 Apr 8;29(7):1669. doi: 10.3390/molecules29071669. Molecules. 2024. PMID: 38611950 Free PMC article. Review.
-
Densities, Viscosities, and Self-Diffusion Coefficients of Octan-1-ol and Related Ether-Alcohols.J Chem Eng Data. 2024 Jul 3;69(8):2688-2699. doi: 10.1021/acs.jced.4c00195. eCollection 2024 Aug 8. J Chem Eng Data. 2024. PMID: 39139987 Free PMC article.
References
-
- Polyethylene Glycol Market Size, Share & Trends Analysis Report by Application (Medical, Personal Care, Industrial), by Region (North America, Europe, Asia Pacific, Row), and Segment Forecasts, 2015–2020. https://www.grandviewresearch.com/industry-analysis/polyethylene-glycol-... (accessed Aug 07, 2021).
-
- Hutanu D. Recent Applications of Polyethylene Glycols (PEGs) and PEG Derivatives. Mod. Chem. Appl. 2014, 2, 1000132.10.4172/2329-6798.1000132. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

