SARS-CoV-2 Antibody Responses to the Ancestral SARS-CoV-2 Strain and Omicron BA.1 and BA.4/BA.5 Variants in Nursing Home Residents After Receipt of Bivalent COVID-19 Vaccine - Ohio and Rhode Island, September-November 2022
- PMID: 36701254
- PMCID: PMC9925133
- DOI: 10.15585/mmwr.mm7204a4
SARS-CoV-2 Antibody Responses to the Ancestral SARS-CoV-2 Strain and Omicron BA.1 and BA.4/BA.5 Variants in Nursing Home Residents After Receipt of Bivalent COVID-19 Vaccine - Ohio and Rhode Island, September-November 2022
Abstract
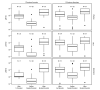
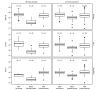
Introduction of monovalent COVID-19 mRNA vaccines in late 2020 helped to mitigate disproportionate COVID-19-related morbidity and mortality in U.S. nursing homes (1); however, reduced effectiveness of monovalent vaccines during the period of Omicron variant predominance led to recommendations for booster doses with bivalent COVID-19 mRNA vaccines that include an Omicron BA.4/BA.5 spike protein component to broaden immune response and improve vaccine effectiveness against circulating Omicron variants (2). Recent studies suggest that bivalent booster doses provide substantial additional protection against SARS-CoV-2 infection and severe COVID-19-associated disease among immunocompetent adults who previously received only monovalent vaccines (3).* The immunologic response after receipt of bivalent boosters among nursing home residents, who often mount poor immunologic responses to vaccines, remains unknown. Serial testing of anti-spike protein antibody binding and neutralizing antibody titers in serum collected from 233 long-stay nursing home residents from the time of their primary vaccination series and including any subsequent booster doses, including the bivalent vaccine, was performed. The bivalent COVID-19 mRNA vaccine substantially increased anti-spike and neutralizing antibody titers against Omicron sublineages, including BA.1 and BA.4/BA.5, irrespective of previous SARS-CoV-2 infection or previous receipt of 1 or 2 booster doses. These data, in combination with evidence of low uptake of bivalent booster vaccination among residents and staff members in nursing homes (4), support the recommendation that nursing home residents and staff members receive a bivalent COVID-19 booster dose to reduce associated morbidity and mortality (2).
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Stefan Gravenstein and David H. Canaday are recipients of support from the U.S. Department of Veterans Affairs and investigator-initiated grants to their universities from the National Institute of Allergy and Infectious Diseases (NIAID) to study influenza vaccine and COVID-19 in the nursing home, Pfizer to study pneumococcal vaccines, and from Sanofi Pasteur and Seqirus to study influenza vaccines. Stefan Gravenstein also performs consulting work for Janssen, Merck, Moderna, Novavax, Pfizer, Sanofi, Seqirus, and Vaxart; has served on the speaker’s bureaus for Seqirus and Sanofi; and was paid to chair data safety monitoring boards from Longeveron and SciClone. David H. Canaday has performed consulting work for Seqirus. Elizabeth M. White reports support from the National Institute on Aging, and membership on the Society for Post-acute and Long-term Care Medicine Workforce Development Committee and on the John Hartford Foundation Moving Forward Coalition Workforce Committee. Jürgen Bosch is the cofounder and Chief Executive Officer of InterRayBio, LLC. Yi Cao, Kerri St. Denis, and Alejandro B. Balazs report support from the Ragon Institute of Massachusetts General Hospital, the Massachusetts Institute of Technology, and Harvard University for equipment used in the current study. Kevin W. McConeghy reports grant support from Sanofi-Pasteur, Sequirus Pharmaceuticals, Genentech, and Janssen, unrelated to the current work. Eleftherios Mylonakis reports institutional support from the Biomedical Advanced Research and Development Authority, NIAID, the National Institute of General Medical Sciences, National Institutes of Health, Leidos Biomedical Research, Inc., Regeneron, Pfizer, Chemic lags/KODA therapeutics, Cidara, the National Cancer Institute, and SciClone Pharmaceuticals, and receipt of consulting fees from Basilea Pharmaceutica International, Ltd. Christopher L. King reports National Cancer Institute support for Early Drivers of Humoral Immunity to SARS-CoV-2 Infections. No other potential conflicts of interest were disclosed.
Figures
References
-
- CDC. Nursing home COVID-19 data dashboard. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://www.cdc.gov/nhsn/covid19/ltc-report-overview.html
-
- CDC. ACIP Evidence to Recommendations (EtR) for use of bivalent COVID-19 vaccine booster doses under an emergency use authorization. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. https://www.cdc.gov/vaccines/acip/recs/grade/covid-19-bivalent-booster-e...
-
- Link-Gelles R, Levy ME, Gaglani M, et al. Effectiveness of 2, 3, and 4 COVID-19 mRNA vaccine doses among immunocompetent adults during periods when SARS-CoV-2 Omicron BA.1 and BA.2/BA.2.12.1 sublineages predominated—VISION Network, 10 states, December 2021–June 2022. MMWR Morb Mortal Wkly Rep 2022;71:931–9. 10.15585/mmwr.mm7129e1 - DOI - PMC - PubMed
-
- CDC. National Healthcare Safety Network: nursing home COVID-19 vaccination data dashboard. Atlanta, GA: Department of Health and Human Services, CDC; 2022. https://www.cdc.gov/nhsn/covid19/ltc-vaccination-dashboard.html
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

