FMRP activity and control of Csw/SHP2 translation regulate MAPK-dependent synaptic transmission
- PMID: 36701299
- PMCID: PMC9879533
- DOI: 10.1371/journal.pbio.3001969
FMRP activity and control of Csw/SHP2 translation regulate MAPK-dependent synaptic transmission
Abstract
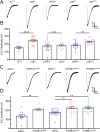
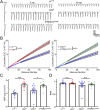
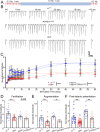
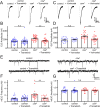
Noonan syndrome (NS) and NS with multiple lentigines (NSML) cognitive dysfunction are linked to SH2 domain-containing protein tyrosine phosphatase-2 (SHP2) gain-of-function (GoF) and loss-of-function (LoF), respectively. In Drosophila disease models, we find both SHP2 mutations from human patients and corkscrew (csw) homolog LoF/GoF elevate glutamatergic transmission. Cell-targeted RNAi and neurotransmitter release analyses reveal a presynaptic requirement. Consistently, all mutants exhibit reduced synaptic depression during high-frequency stimulation. Both LoF and GoF mutants also show impaired synaptic plasticity, including reduced facilitation, augmentation, and post-tetanic potentiation. NS/NSML diseases are characterized by elevated MAPK/ERK signaling, and drugs suppressing this signaling restore normal neurotransmission in mutants. Fragile X syndrome (FXS) is likewise characterized by elevated MAPK/ERK signaling. Fragile X Mental Retardation Protein (FMRP) binds csw mRNA and neuronal Csw protein is elevated in Drosophila fragile X mental retardation 1 (dfmr1) nulls. Moreover, phosphorylated ERK (pERK) is increased in dfmr1 and csw null presynaptic boutons. We find presynaptic pERK activation in response to stimulation is reduced in dfmr1 and csw nulls. Trans-heterozygous csw/+; dfmr1/+ recapitulate elevated presynaptic pERK activation and function, showing FMRP and Csw/SHP2 act within the same signaling pathway. Thus, a FMRP and SHP2 MAPK/ERK regulative mechanism controls basal and activity-dependent neurotransmission strength.
Copyright: © 2023 Leahy et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
PTPN11/Corkscrew Activates Local Presynaptic Mapk Signaling to Regulate Synapsin, Synaptic Vesicle Pools, and Neurotransmission Strength, with a Dual Requirement in Neurons and Glia.J Neurosci. 2024 Apr 24;44(17):e1077232024. doi: 10.1523/JNEUROSCI.1077-23.2024. J Neurosci. 2024. PMID: 38471782 Free PMC article.
-
Metabotropic glutamate receptor-mediated use-dependent down-regulation of synaptic excitability involves the fragile X mental retardation protein.J Neurophysiol. 2009 Feb;101(2):672-87. doi: 10.1152/jn.90953.2008. Epub 2008 Nov 26. J Neurophysiol. 2009. PMID: 19036865 Free PMC article.
-
Temporal requirements of the fragile X mental retardation protein in the regulation of synaptic structure.Development. 2008 Aug;135(15):2637-48. doi: 10.1242/dev.022244. Epub 2008 Jun 25. Development. 2008. PMID: 18579676 Free PMC article.
-
dFmr1 Plays Roles in Small RNA Pathways of Drosophila melanogaster.Int J Mol Sci. 2017 May 16;18(5):1066. doi: 10.3390/ijms18051066. Int J Mol Sci. 2017. PMID: 28509881 Free PMC article. Review.
-
Fragile X Mental Retardation Protein Regulates Activity-Dependent Membrane Trafficking and Trans-Synaptic Signaling Mediating Synaptic Remodeling.Front Mol Neurosci. 2018 Jan 12;10:440. doi: 10.3389/fnmol.2017.00440. eCollection 2017. Front Mol Neurosci. 2018. PMID: 29375303 Free PMC article. Review.
Cited by
-
Rasopathy-Associated Mutation Ptpn11D61Y has Age-Dependent Effect on Synaptic Vesicle Recycling.Cell Mol Neurobiol. 2024 Nov 21;44(1):77. doi: 10.1007/s10571-024-01505-1. Cell Mol Neurobiol. 2024. PMID: 39570442 Free PMC article.
-
PTPN11/Corkscrew Activates Local Presynaptic Mapk Signaling to Regulate Synapsin, Synaptic Vesicle Pools, and Neurotransmission Strength, with a Dual Requirement in Neurons and Glia.J Neurosci. 2024 Apr 24;44(17):e1077232024. doi: 10.1523/JNEUROSCI.1077-23.2024. J Neurosci. 2024. PMID: 38471782 Free PMC article.
-
Muscle cofilin alters neuromuscular junction postsynaptic development to strengthen functional neurotransmission.Development. 2024 Jul 1;151(13):dev202558. doi: 10.1242/dev.202558. Epub 2024 Jul 8. Development. 2024. PMID: 38869008 Free PMC article.
-
Experience-dependent MAPK/ERK signaling in glia regulates critical period remodeling of synaptic glomeruli.Cell Signal. 2024 Aug;120:111224. doi: 10.1016/j.cellsig.2024.111224. Epub 2024 May 11. Cell Signal. 2024. PMID: 38740233 Free PMC article.
-
Neuron-to-glia signaling drives critical period experience-dependent synapse pruning.Sci Rep. 2025 Jul 16;15(1):25744. doi: 10.1038/s41598-025-11528-3. Sci Rep. 2025. PMID: 40670566 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

