Spin Hyperpolarization in Modern Magnetic Resonance
- PMID: 36701528
- PMCID: PMC9951229
- DOI: 10.1021/acs.chemrev.2c00534
Spin Hyperpolarization in Modern Magnetic Resonance
Abstract
Magnetic resonance techniques are successfully utilized in a broad range of scientific disciplines and in various practical applications, with medical magnetic resonance imaging being the most widely known example. Currently, both fundamental and applied magnetic resonance are enjoying a major boost owing to the rapidly developing field of spin hyperpolarization. Hyperpolarization techniques are able to enhance signal intensities in magnetic resonance by several orders of magnitude, and thus to largely overcome its major disadvantage of relatively low sensitivity. This provides new impetus for existing applications of magnetic resonance and opens the gates to exciting new possibilities. In this review, we provide a unified picture of the many methods and techniques that fall under the umbrella term "hyperpolarization" but are currently seldom perceived as integral parts of the same field. Specifically, before delving into the individual techniques, we provide a detailed analysis of the underlying principles of spin hyperpolarization. We attempt to uncover and classify the origins of hyperpolarization, to establish its sources and the specific mechanisms that enable the flow of polarization from a source to the target spins. We then give a more detailed analysis of individual hyperpolarization techniques: the mechanisms by which they work, fundamental and technical requirements, characteristic applications, unresolved issues, and possible future directions. We are seeing a continuous growth of activity in the field of spin hyperpolarization, and we expect the field to flourish as new and improved hyperpolarization techniques are implemented. Some key areas for development are in prolonging polarization lifetimes, making hyperpolarization techniques more generally applicable to chemical/biological systems, reducing the technical and equipment requirements, and creating more efficient excitation and detection schemes. We hope this review will facilitate the sharing of knowledge between subfields within the broad topic of hyperpolarization, to help overcome existing challenges in magnetic resonance and enable novel applications.
Conflict of interest statement
The authors declare the following competing financial interest(s): Eduard Y. Chekmenev has a stake of ownership in XeUS Technologies Ltd.
Figures



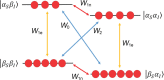
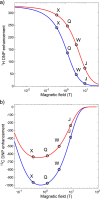
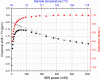
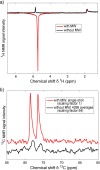





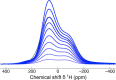

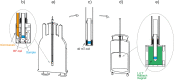
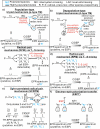

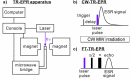
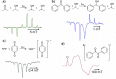
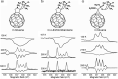
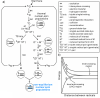
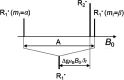
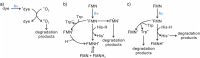
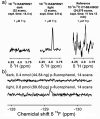
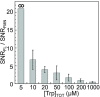
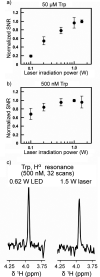

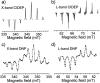
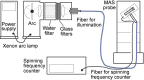
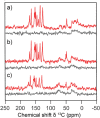

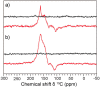







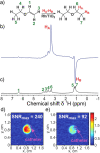

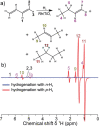

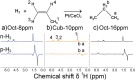
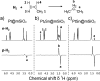
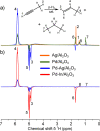
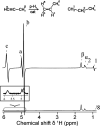
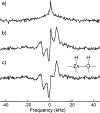
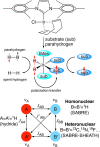


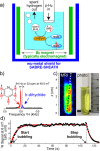

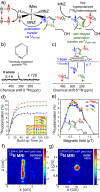
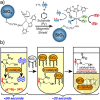
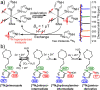

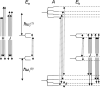
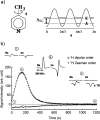
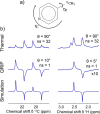
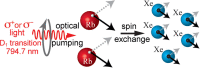




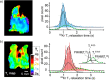

References
-
- Moser E.; Laistler E.; Schmitt F.; Kontaxis G. Ultra-High Field NMR and MRI - the Role of Magnet Technology to Increase Sensitivity and Specificity. Frontiers in Physics 2017, 5, 33. 10.3389/fphy.2017.00033. - DOI
-
- Burueva D. B.; Eills J.; Blanchard J. W.; Garcon A.; Picazo-Frutos R.; Kovtunov K. V.; Koptyug I. V.; Budker D. Chemical Reaction Monitoring Using Zero-Field Nuclear Magnetic Resonance Enables Study of Heterogeneous Samples in Metal Containers. Angew. Chem., Int. Ed. 2020, 59, 17026–17032. 10.1002/anie.202006266. - DOI - PMC - PubMed
-
- Blanchard J. W.; Sjolander T. F.; King J. P.; Ledbetter M. P.; Levine E. H.; Bajaj V. S.; Budker D.; Pines A. Measurement of Untruncated Nuclear Spin Interactions via Zero- to Ultralow-Field Nuclear Magnetic Resonance. Phys. Rev. B 2015, 92, 220202. 10.1103/PhysRevB.92.220202. - DOI

