Dioxygen Binding Is Controlled by the Protein Environment in Non-heme FeII and 2-Oxoglutarate Oxygenases: A Study on Histone Demethylase PHF8 and an Ethylene-Forming Enzyme
- PMID: 36701641
- PMCID: PMC10305803
- DOI: 10.1002/chem.202300138
Dioxygen Binding Is Controlled by the Protein Environment in Non-heme FeII and 2-Oxoglutarate Oxygenases: A Study on Histone Demethylase PHF8 and an Ethylene-Forming Enzyme
Abstract
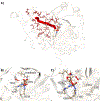
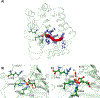
This study investigates dioxygen binding and 2-oxoglutarate (2OG) coordination by two model non-heme FeII /2OG enzymes: a class 7 histone demethylase (PHF8) that catalyzes the hydroxylation of its H3K9me2 histone substrate leading to demethylation reactivity and the ethylene-forming enzyme (EFE), which catalyzes two competing reactions of ethylene generation and substrate l-Arg hydroxylation. Although both enzymes initially bind 2OG by using an off-line 2OG coordination mode, in PHF8, the substrate oxidation requires a transition to an in-line mode, whereas EFE is catalytically productive for ethylene production from 2OG in the off-line mode. We used classical molecular dynamics (MD), quantum mechanics/molecular mechanics (QM/MM) MD and QM/MM metadynamics (QM/MM-MetD) simulations to reveal that it is the dioxygen binding process and, ultimately, the protein environment that control the formation of the in-line FeIII -OO⋅- intermediate in PHF8 and the off-line FeIII -OO⋅- intermediate in EFE.
Keywords: QM/MM metadynamics; dioxygen diffusion; ethylene-forming enzymes; histone demethylation; molecular dynamics.
© 2023 Wiley-VCH GmbH.
Figures


References
-
- Schofield CJ, Hausinger R, 2-Oxoglutarate-Dependent Oxygenases (Eds.: Schofield CJ, Hausinger R), Metallobiology; Royal Society Of Chemistry, Cambridge, 2015, pp. 1–58.

