Head-to-head comparison of four COVID-19 vaccines on platelet activation, coagulation and inflammation. The TREASURE study
- PMID: 36702064
- PMCID: PMC9846886
- DOI: 10.1016/j.thromres.2023.01.015
Head-to-head comparison of four COVID-19 vaccines on platelet activation, coagulation and inflammation. The TREASURE study
Abstract
Introduction: Studies exploring alterations in blood coagulation and platelet activation induced by COVID-19 vaccines are not concordant. We aimed to assess the impact of four COVID-19 vaccines on platelet activation, coagulation, and inflammation considering also the immunization dose and the history of SARS-CoV-2 infection.
Methods: TREASURE study enrolled 368 consecutive subjects (161 receiving viral vector vaccines -ChAdOx1-S/Vaxzevria or Janssen- and 207 receiving mRNA vaccines -Comirnaty/Pfizer-BioNTech or Spikevax/Moderna). Blood was collected the day before and 8 ± 2 days after the vaccination. Platelet activation markers (P-selectin, aGPIIbIIIa and Tissue Factor expression; number of platelet-monocyte and -granulocyte aggregates) and microvesicle release were analyzed by flow cytometry. Platelet thrombin generation (TG) capacity was measured using the Calibrated Automated Thrombogram. Plasma coagulation and inflammation markers and immune response were evaluated by ELISA.
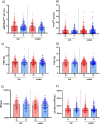
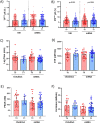
Results: Vaccination did not induce platelet activation and microvesicle release. IL-6 and CRP levels (+30%), D-dimer, fibrinogen and F1+2 (+13%, +3.7%, +4.3%, respectively) but not TAT levels significantly increased upon immunization with all four vaccines, with no difference among them and between first and second dose. An overall minor post-vaccination reduction of aPC, TM and TFPI, all possibly related to endothelial function, was observed. No anti-PF4 seroconversion was observed.
Conclusion: This study showed that the four COVID-19 vaccines administered to a large population sample induce a transient inflammatory response, with no onset of platelet activation. The minor changes in clotting activation and endothelial function might be potentially involved at a population level in explaining the very rare venous thromboembolic complications of COVID-19 vaccination.
Keywords: COVID-19 vaccine; Coagulation; Endothelial dysfunction; Inflammation; Platelet activation; Thrombosis.
Copyright © 2023. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Inflammation and Platelet Activation After COVID-19 Vaccines - Possible Mechanisms Behind Vaccine-Induced Immune Thrombocytopenia and Thrombosis.Front Immunol. 2021 Nov 23;12:779453. doi: 10.3389/fimmu.2021.779453. eCollection 2021. Front Immunol. 2021. PMID: 34887867 Free PMC article. Clinical Trial.
-
SARS-CoV-2 vaccines are not associated with hypercoagulability in apparently healthy people.Res Pract Thromb Haemost. 2023 Jan;7(1):100002. doi: 10.1016/j.rpth.2022.100002. Epub 2022 Nov 25. Res Pract Thromb Haemost. 2023. PMID: 36448024 Free PMC article.
-
Anti-severe acute respiratory syndrome coronavirus-2 adenoviral-vector vaccines trigger subclinical antiplatelet autoimmunity and increase of soluble platelet activation markers.Br J Haematol. 2022 Jul;198(2):257-266. doi: 10.1111/bjh.18245. Epub 2022 May 16. Br J Haematol. 2022. PMID: 35577507
-
The role of anti-platelet factor 4 antibodies and platelet activation tests in patients with vaccine-induced immune thrombotic thrombocytopenia: Brief report on a comparison of the laboratory diagnosis and literature review.Clin Chim Acta. 2022 Apr 1;529:42-45. doi: 10.1016/j.cca.2022.02.003. Epub 2022 Feb 12. Clin Chim Acta. 2022. PMID: 35167842 Free PMC article. Review.
-
COVID-19 vaccines: comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines.Eur Rev Med Pharmacol Sci. 2021 Feb;25(3):1663-1669. doi: 10.26355/eurrev_202102_24877. Eur Rev Med Pharmacol Sci. 2021. PMID: 33629336 Review.
Cited by
-
Coagulation and inflammatory response after intramuscular or intradermal mRNA-1273 SARS-CoV-2 vaccine: secondary analysis of a randomized trial.Res Pract Thromb Haemost. 2024 Apr 24;8(3):102419. doi: 10.1016/j.rpth.2024.102419. eCollection 2024 Mar. Res Pract Thromb Haemost. 2024. PMID: 38779329 Free PMC article.
-
CCL20 chemokine and other proinflammatory markers after Ad26.COV2.S vaccination.Biochem Med (Zagreb). 2024 Oct 15;34(3):030706. doi: 10.11613/BM.2024.030706. Biochem Med (Zagreb). 2024. PMID: 39435167 Free PMC article.
-
The Lung and Winding Road: Inflammation-Driven Platelet Reactivity in Long COVID.JACC Basic Transl Sci. 2025 Jan 27;10(1):40-42. doi: 10.1016/j.jacbts.2024.11.013. eCollection 2025 Jan. JACC Basic Transl Sci. 2025. PMID: 39958471 Free PMC article.
-
Hemostatic changes following COVID-19 vaccination: Do they promote a pro-thrombotic state?Hum Vaccin Immunother. 2025 Dec;21(1):2439627. doi: 10.1080/21645515.2024.2439627. Epub 2024 Dec 19. Hum Vaccin Immunother. 2025. PMID: 39699990 Free PMC article.
-
The impact of Gam-COVID-Vac, an Adv5/Adv26 COVID-19 vaccine, on the biomarkers of endothelial function, coagulation and platelet activation.PLoS One. 2023 Oct 18;18(10):e0293074. doi: 10.1371/journal.pone.0293074. eCollection 2023. PLoS One. 2023. PMID: 37851684 Free PMC article.
References
-
- Ostrowski S.R., Sogaard O.S., Tolstrup M., Staerke N.B., Lundgren J., Ostergaard L., Hvas A.M. Inflammation and platelet activation after COVID-19 vaccines - possible mechanisms behind vaccine-induced immune thrombocytopenia and thrombosis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.779453. - DOI - PMC - PubMed
-
- Petito E., Colonna E., Falcinelli E., Mezzasoma A.M., Cesari E., Giglio E., Fiordi T., Almerigogna F., Villa A., Gresele P. Anti-severe acute respiratory syndrome coronavirus-2 adenoviral-vector vaccines trigger subclinical antiplatelet autoimmunity and increase of soluble platelet activation markers. Br. J. Haematol. 2022;198:257–266. doi: 10.1111/bjh.18245. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

