Cabozantinib plus durvalumab in advanced gastroesophageal cancer and other gastrointestinal malignancies: Phase Ib CAMILLA trial results
- PMID: 36702123
- PMCID: PMC9975105
- DOI: 10.1016/j.xcrm.2023.100916
Cabozantinib plus durvalumab in advanced gastroesophageal cancer and other gastrointestinal malignancies: Phase Ib CAMILLA trial results
Abstract
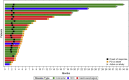
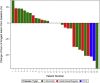
This is the phase Ib part of the phase I/II CAMILLA trial evaluating cabozantinib plus durvalumab in advanced chemo-refractory proficient mismatch repair or microsatellite stable (pMMR/MSS) gastrointestinal malignancies including gastric/gastroesophageal junction/esophageal (G/GEJ/E) adenocarcinoma, colorectal cancer (CRC), and hepatocellular carcinoma (HCC). Thirty-five patients are enrolled. There are no observed dose-limiting toxicities during dose escalation. The overall grade 3/4 treatment-related adverse event rate is 34%. Among evaluable patients (n = 30), the objective response rate (ORR) is 30%, disease control rate (DCR) 83.3%, 6-month progression-free survival (PFS) 36.7%, median PFS 4.5 months, and median overall survival (OS) 8.7 months. Responses are seen in 4 of 17, 3 of 10, and 2 of 3 patients with CRC, G/GEJ/E adenocarcinoma, and HCC, respectively. Participants with a PD-L1 combined positive score (CPS) ≥5 have numerically higher ORR, PFS, and OS. Cabozantinib plus durvalumab demonstrates a tolerable safety profile and potential efficacy in previously treated advanced pMMR/MSS gastrointestinal malignancies.
Keywords: cabozantinib; colorectal cancer; durvalumab; gastroesophageal cancer; hepatocellular carcinoma.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A.S. reports research grants (to institution) from AstraZeneca, Bristol Myers Squibb, Merck, Clovis, Exelixis, Actuate Therapeutics, Incyte Corporation, Daiichi Sankyo, Amgen, Innovent Biologics, Dragonfly Therapeutics, KAHR Medical, and Biontech and advisory board fees from AstraZeneca, Bristol Myers Squibb, Exelixis, Pfizer, and Daiichi Sankyo. R.A.-R. reports research grants (to institution) from AstraZeneca, Bayor, Merck, Bristol Myers Squibb, Exelixis, and Eureka Therapeutics and stock ownership in Actinium Pharmaceuticals and Seagen. A.K. reports research funding (to institution) from Astellas, Tesaro, and Bavarian Nordic. J.B. reports research grants (to institution) from Astellas, Chungchun Intellicrown, Impact Therapeutics, Poseida Therapeutics, Takeda Oncology, and Genome Co. and consultant fees (to institution) from Sanofi. A.K.G. reports research funding from Predicine and VITRAC Therapeutics, is a co-founder of Sinochips Diagnostics, and serves as a scientific advisory board member to Biovica, Clara Biotech, and Sinochips Diagnostics. S.W. reports stock/other ownership in Horizon Therapeutics, Iovance Biotherapeutics, and Merus and institutional research funding from Aleon Pharma, Astellas Pharma, Bayer Health, Bristol Myers Squibb, Daiichi Sankyo, EMD Serono, Merck Serono, Nektar, Novartis, Pharmacyclics, AbbVie, Regeneron, Rogosin Institute, Sanofi, Seagen, and Sotio.
Figures


References
-
- Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. - DOI - PMC - PubMed
-
- Tan E., Sahin I.H. Defining the current role of immune checkpoint inhibitors in the treatment of mismatch repair-deficient/microsatellite stability-high colorectal cancer and shedding light on future approaches. Expet Rev. Gastroenterol. Hepatol. 2021;15:735–742. doi: 10.1080/17474124.2021.1886077. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

