Ferroptosis in acute kidney injury following crush syndrome: A novel target for treatment
- PMID: 36702249
- PMCID: PMC10703611
- DOI: 10.1016/j.jare.2023.01.016
Ferroptosis in acute kidney injury following crush syndrome: A novel target for treatment
Abstract
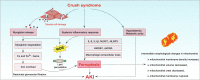
Background: Crush syndrome (CS) is a kind of traumatic and ischemic injury that seriously threatens life after prolonged compression. It is characterized by systemic inflammatory reaction, myoglobinuria, hyperkalemia and acute kidney injury (AKI). Especially AKI, it is the leading cause of death from CS. There are various cell death forms in AKI, among which ferroptosis is a typical form of cell death. However, the role of ferroptosis has not been fully revealed in CS-AKI.
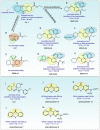
Aim of review: This review aimed to summarize the evidence of ferroptosis in CS-AKI and its related molecular mechanism, discuss the therapeutic significance of ferroptosis in CS-AKI, and open up new ideas for the treatment of CS-AKI.
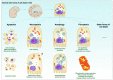
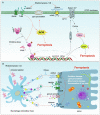
Key scientific concepts of review: One of the main pathological manifestations of CS-AKI is renal tubular epithelial cell dysfunction and cell death, which has been attributed to massive deposition of myoglobin. Large amounts of myoglobin released from damaged muscle deposited in the renal tubules, impeding the normal renal tubules function and directly damaging the tubules with oxidative stress and elevated iron levels. Lipid peroxidation damage and iron overload are the distinguishing features of ferroptosis. Moreover, high levels of pro-inflammatory cytokines and damage-associated molecule pattern molecules (HMGB1, double-strand DNA, and macrophage extracellular trap) in renal tissue have been shown to promote ferroptosis. However, how ferroptosis occurs in CS-AKI and whether it can be a therapeutic target remains unclear. In our current work, we systematically reviewed the occurrence and underlying mechanism of ferroptosis in CS-AKI.
Keywords: Acute kidney injury; Crush syndrome; Drug development; Ferroptosis; Myoglobin; Systemic inflammatory responses.
Copyright © 2023. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

