Identification of niclosamide as a novel antiviral agent against porcine epidemic diarrhea virus infection by targeting viral internalization
- PMID: 36702255
- PMCID: PMC10176444
- DOI: 10.1016/j.virs.2023.01.008
Identification of niclosamide as a novel antiviral agent against porcine epidemic diarrhea virus infection by targeting viral internalization
Abstract
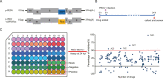
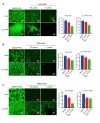
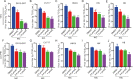
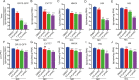
Porcine epidemic diarrhea virus (PEDV), an enteropathogenic coronavirus, has catastrophic impacts on the global pig industry. However, there remain no effective drugs against PEDV infection. In this study, we utilized a recombinant PEDV expressing renilla luciferase (PEDV-Rluc) to screen potential anti-PEDV agents from an FDA-approved drug library in Vero cells. Four compounds were identified that significantly decreased luciferase activity of PEDV-Rluc. Among them, niclosamide was further characterized because it exhibited the most potent antiviral activity with the highest selectivity index. It can efficiently inhibit viral RNA synthesis, protein expression and viral progeny production of classical and variant PEDV strains in a dose-dependent manner. Time of addition assay showed that niclosamide exhibited potent anti-PEDV activity when added simultaneously with or after virus infection. Furthermore, niclosamide significantly inhibited the entry stage of PEDV infection by affecting viral internalization rather than viral attachment to cells. In addition, a combination with other small molecule inhibitors of endosomal acidification enhanced the anti-PEDV effect of niclosamide in vitro. Taken together, these findings suggested that niclosamide is a novel antiviral agent that might provide a basis for the development of novel drug therapies against PEDV and other related pathogenic coronavirus infections.
Keywords: Antiviral; Coronavirus; Endocytosis; Host-targeted antivirals; Niclosamide (NIC); Porcine epidemic diarrhea virus (PEDV); Virus entry.
Copyright © 2023 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Evaluating anti-viral effect of Ivermectin on porcine epidemic diarrhea virus and analyzing the related genes and signaling pathway by RNA-seq in vitro.Virology. 2023 Oct;587:109877. doi: 10.1016/j.virol.2023.109877. Epub 2023 Sep 4. Virology. 2023. PMID: 37688922
-
Antiviral activity of flavonol against porcine epidemic diarrhea virus.Virology. 2024 Sep;597:110128. doi: 10.1016/j.virol.2024.110128. Epub 2024 Jun 1. Virology. 2024. PMID: 38861876
-
Antiviral effect of lithium chloride on porcine epidemic diarrhea virus in vitro.Res Vet Sci. 2018 Jun;118:288-294. doi: 10.1016/j.rvsc.2018.03.002. Epub 2018 Mar 5. Res Vet Sci. 2018. PMID: 29547727 Free PMC article.
-
Harnessing Nanohybridized Niclosamide for Precision Mpox Therapeutics.Adv Healthc Mater. 2025 May;14(14):e2404818. doi: 10.1002/adhm.202404818. Epub 2025 Feb 23. Adv Healthc Mater. 2025. PMID: 39988865 Free PMC article. Review.
-
Pharmacological advances and therapeutic applications of niclosamide in cancer and other diseases.Eur J Med Chem. 2025 Jun 5;290:117527. doi: 10.1016/j.ejmech.2025.117527. Epub 2025 Mar 20. Eur J Med Chem. 2025. PMID: 40153934 Review.
Cited by
-
Pemetrexed alleviates piglet diarrhea by blocking the interaction between porcine epidemic diarrhea virus nucleocapsid protein and Ezrin.J Virol. 2024 Jan 23;98(1):e0162523. doi: 10.1128/jvi.01625-23. Epub 2023 Dec 12. J Virol. 2024. PMID: 38084960 Free PMC article.
-
Transforming Niclosamide through Nanotechnology: A Promising Approach for Long COVID Management.Small. 2025 Jul;21(27):e2410345. doi: 10.1002/smll.202410345. Epub 2025 May 19. Small. 2025. PMID: 40384184 Free PMC article. Review.
-
Recent Advances on Targeting Proteases for Antiviral Development.Viruses. 2024 Feb 27;16(3):366. doi: 10.3390/v16030366. Viruses. 2024. PMID: 38543732 Free PMC article. Review.
-
Drug repurposing screens identify Tubercidin as a potent antiviral agent against porcine nidovirus infections.Virus Res. 2024 Jan 2;339:199275. doi: 10.1016/j.virusres.2023.199275. Epub 2023 Nov 30. Virus Res. 2024. PMID: 38008220 Free PMC article.
-
Calmodulin-like 5 promotes PEDV replication by regulating late-endosome synthesis and innate immune response.Virol Sin. 2024 Jun;39(3):501-512. doi: 10.1016/j.virs.2024.05.006. Epub 2024 May 23. Virol Sin. 2024. PMID: 38789039 Free PMC article.
References
-
- Braga L., Ali H., Secco I., Chiavacci E., Neves G., Goldhill D., Penn R., Jimenez-Guardeño J.M., Ortega-Prieto A.M., Bussani R., Cannatà A., Rizzari G., Collesi C., Schneider E., Arosio D., Shah A.M., Barclay W.S., Malim M.H., Burrone J., Giacca M. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature. 2021;594:88–93. - PMC - PubMed
-
- Cao L., Ge X., Gao Y., Ren Y., Ren X., Li G. Porcine epidemic diarrhea virus infection induces NF-κB activation through the TLR2, TLR3 and TLR9 pathways in porcine intestinal epithelial cells. J. Gen. Virol. 2015;96:1757–1767. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

