Murine toxicology and pharmacokinetics of lead next generation galeterone analog, VNPP433-3β
- PMID: 36702363
- PMCID: PMC9998351
- DOI: 10.1016/j.steroids.2023.109184
Murine toxicology and pharmacokinetics of lead next generation galeterone analog, VNPP433-3β
Abstract
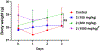
VNPP433-3β (compound 2, (3β-(1H-imidazole-1-yl)-17-(1H-benzimidazole-1-yl)-androsta-5,16-diene), a multitarget anticancer agent has emerged as our lead next generation galeterone analogs (NGGA). Compound 2 is currently in development as potential new therapeutic for prostate and pancreatic cancers. The preliminary toxicity study reveals that the compound 2 was better tolerated by the normal male CD-1 mice than the male Nude mice. The maximum tolerated dose (MTD) in the Nude mice was estimated to be between 25 < 50 mg/kg. After oral dosing of compound 2 to male and female rats, the plasma concentration versus time curves were very consistent between animals and the AUClast increased with dose. Many plasmas concentration versus time curves profiles were nearly flat over 24 hr., suggesting extended absorption from the GI tract. Consequently, reliable values for half-life and AUCinf were not determined. Calculated oral bioavailability (using oral AUClast and excluding the outlier IV animal) ranged from 32 to 47 %. This should be considered a minimum value since the contribution to true AUC beyond 24 hr. is clearly not zero. Clearly, these toxicology and pharmacokinetics parameters pave the way for understanding the anticancer pharmacological actions and provide a meaningful basis for further preclinical development and eventual clinical development.
Keywords: Anti-prostate cancer agent; Oral bioavailability; Pharmacokinetics; Toxicology; VNPP433-3β (2).
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Vincent C. O. Njar is the lead inventor of VNPP433-3β, the patents and technologies thereof are owned by the University of Maryland, Baltimore. Puranik Purushottamachar is a co-inventor of VNPP433-3β. The other authors declare no potential conflict of interest.
Figures
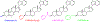
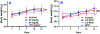
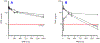

References
-
- Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y, A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth, Cancer Res 69(6) (2009) 2305–13. - PMC - PubMed
-
- Mostaghel EA, Marck BT, Plymate SR, Vessella RL, Balk S, Matsumoto AM, Nelson PS, Montgomery RB, Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: induction of steroidogenesis and androgen receptor splice variants, Clin Cancer Res 17(18) (2011) 5913–25. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

