Waning of 2-Dose BNT162b2 and mRNA-1273 Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection Accounting for Depletion-of-Susceptibles Bias
- PMID: 36702469
- PMCID: PMC10236522
- DOI: 10.1093/aje/kwad017
Waning of 2-Dose BNT162b2 and mRNA-1273 Vaccine Effectiveness Against Symptomatic SARS-CoV-2 Infection Accounting for Depletion-of-Susceptibles Bias
Abstract
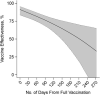
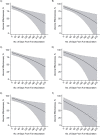
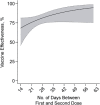
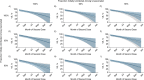
Concerns about the duration of protection conferred by coronavirus disease 2019 (COVID-19) vaccines have arisen in postlicensure evaluations. "Depletion of susceptibles," a bias driven by differential accrual of infection among vaccinated and unvaccinated individuals, may obscure vaccine effectiveness (VE) estimates, hindering interpretation. We enrolled California residents who received molecular SARS-CoV-2 tests in a matched, test-negative design, case-control study to estimate VE of mRNA-based COVID-19 vaccines between February 23 and December 5, 2021. We analyzed waning protection following 2 vaccine doses using conditional logistic regression models. Additionally, we used data from a population-based serological study to adjust for "depletion-of-susceptibles" bias and estimated VE for 3 doses, by time since second dose receipt. Pooled VE of BNT162b2 and mRNA-1273 against symptomatic SARS-CoV-2 infection was 91.3% (95% confidence interval (CI): 83.8, 95.4) at 14 days after second-dose receipt and declined to 50.8% (95% CI: 19.7, 69.8) at 7 months. Adjusting for depletion-of-susceptibles bias, we estimated VE of 53.2% (95% CI: 23.6, 71.2) at 7 months after primary mRNA vaccination series. A booster dose of BN162b2 or mRNA-1273 increased VE to 95.0% (95% CI: 82.8, 98.6). These findings confirm that observed waning of protection is not attributable to epidemiologic bias and support ongoing efforts to administer additional vaccine doses to mitigate burden of COVID-19.
Keywords: COVID-19; SARS-CoV-2; bias; depletion of susceptibles; vaccination; vaccine effectiveness.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Johns Hopkins Bloomberg School of Public Health.
Figures
References
-
- Centers for Disease Control and Prevention . COVIDVaxView. 2021; https://www.cdc.gov/vaccines/imz-managers/coverage/covidvaxview/index.html. Accessed November 30, 2021.

