The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Antigen Testing (January 2023)
- PMID: 36702617
- PMCID: PMC12098014
- DOI: 10.1093/cid/ciad032
The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Antigen Testing (January 2023)
Abstract
Immunoassays designed to detect SARS-CoV-2 protein antigens (Ag) are commonly used to diagnose COVID-19. The most widely used tests are lateral flow assays that generate results in approximately 15 minutes for diagnosis at the point-of-care. Higher throughput, laboratory-based SARS-CoV-2 Ag assays have also been developed. The number of commercially available SARS-CoV-2 Ag detection tests has increased rapidly, as has the COVID-19 diagnostic literature. The Infectious Diseases Society of America (IDSA) convened an expert panel to perform a systematic review of the literature and develop best-practice guidance related to SARS-CoV-2 Ag testing. This guideline is an update to the third in a series of frequently updated COVID-19 diagnostic guidelines developed by the IDSA. IDSA's goal was to develop evidence-based recommendations or suggestions that assist clinicians, clinical laboratories, patients, public health authorities, administrators, and policymakers in decisions related to the optimal use of SARS-CoV-2 Ag tests in both medical and nonmedical settings. A multidisciplinary panel of infectious diseases clinicians, clinical microbiologists, and experts in systematic literature review identified and prioritized clinical questions related to the use of SARS-CoV-2 Ag tests. A review of relevant, peer-reviewed published literature was conducted through 1 April 2022. Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology was used to assess the certainty of evidence and make testing recommendations. The panel made 10 diagnostic recommendations that address Ag testing in symptomatic and asymptomatic individuals and assess single versus repeat testing strategies. US Food and Drug Administration (FDA) SARS-CoV-2 Ag tests with Emergency Use Authorization (EUA) have high specificity and low to moderate sensitivity compared with nucleic acid amplification testing (NAAT). Ag test sensitivity is dependent on the presence or absence of symptoms and, in symptomatic patients, on timing of testing after symptom onset. In most cases, positive Ag results can be acted upon without confirmation. Results of point-of-care testing are comparable to those of laboratory-based testing, and observed or unobserved self-collection of specimens for testing yields similar results. Modeling suggests that repeat Ag testing increases sensitivity compared with testing once, but no empirical data were available to inform this question. Based on these observations, rapid RT-PCR or laboratory-based NAAT remain the testing methods of choice for diagnosing SARS-CoV-2 infection. However, when timely molecular testing is not readily available or is logistically infeasible, Ag testing helps identify individuals with SARS-CoV-2 infection. Data were insufficient to make a recommendation about the utility of Ag testing to guide release of patients with COVID-19 from isolation. The overall quality of available evidence supporting use of Ag testing was graded as very low to moderate.
Keywords: SARS-CoV-2; diagnostic test performance; diagnostic testing; rapid antigen tests; systematic review.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest. The following list displays what has been reported to the IDSA. To provide thorough transparency, the IDSA requires full disclosure of all relationships, regardless of relevancy to the guideline topic. Evaluation of such relationships as potential conflicts of interest is determined by a review process which includes assessment by the Board of Directors’ liaison to the Standards and Practice Guideline Committee and, if necessary, the Conflicts of Interest (COI) and Ethics Committee. The assessment of disclosed relationships for possible COI is based on the relative weight of the financial relationship (ie, monetary amount) and the relevance of the relationship (ie, the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The reader of these guidelines should be mindful of this when the list of disclosures is reviewed. M. K. H. serves on a clinical adjudication panel for Sanofi; receives research funding from the Centers for Disease Control and Prevention (CDC) (grant and contract for investigator-initiated research) and the CDC Foundation (contract for investigator-initiated research); serves on the Society for Healthcare Epidemiology of America (SHEA) Board of Directors and Chair of the SHEA Education and Research Foundation (President of SHEA Board of Directors 2021; President, SHEA Foundation 2022); received other numerations from Sage, Medline, and Molnylycke; and served as Chair of the IDSA Diagnostics Committee; and reports participation as a member on a Clinical Adjudication Panel for investigational COVID-19 vaccine made by Sanofi. K. E. H. served as an advisor to Quidel, BioFire, Pfizer, and Takeda; received other numerations from Quidel, Pfizer, and Takeda; served as an editor to the American Society of Microbiology (ASM) and member of Clinical and Laboratory Standards Institute Antifungal Committee; received research funding from the National Institutes of Health (NIH); and served on the exam committee for the American Board of Internal Medicine, and associate editor for Open Forum Infectious Diseases. J. A. E. serves as a consultant for Sanofi Pasteur, Pfizer, Moderna, and AstraZeneca; serves as an advisor/consultant for Meissa Vaccines; receives research funding from the CDC, Pfizer, Brotman Baty Research Institute, Merck, Novavax, GlaxoSmithKline (GSK), and AstraZeneca; served as an advisor to Teva Pharmaceuticals; and served as member of Pediatric Infectious Diseases Society (PIDS) Publication Committee and Transplant ID Committee; and reports support for travel to the 2022 European Society for Paediatric Infectious Diseases (ESPID) meeting from ESPID/AstraZeneca. M. J. L. serves as an advisor for Sanofi, Seqirus, Medicago, GSK, Janssen, Novavax, Pfizer, and MD Brief; receives research funding from the Canadian Institutes of Health Research, World Health Organization (WHO), and Medical Research Council (United Kingdom); has received an in-kind supply of vaccine from Sanofi; has been paid for expert testimony on institutional and workplace vaccine policy; and has served on the Data Safety and Monitoring Board (DSMB) for CanSino Biologics and an advisor to Merck. R. P. has a patent on Bordetella pertussis/parapertussis PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued; serves as consultant to PhAST, Torus Biosystems, Day Zero Diagnostics, Mammoth Biosciences, Netflix, Abbott Laboratories, Oxford Nanopore Technologies, CARB-X, Qvella, and HealthTrackRx (monies from PhAST, Torus Biosystems, Day Zero Diagnostics, Mammoth Biosciences, and HealthTrackRx paid to Mayo Clinic); receives other numeration (honoraria) from National Board of Medical Examiners, UpToDate, and the Infectious Disease Board Review Course; received grants from CD Diagnostics, Merck, Hutchison Biofilm Medical Solutions, Accelerate, ContraFect, TenNor Therapeutics Limited, Shionogi, NIH, BioFire, Adaptive Phage Therapeutics, National Science Foundation, and the Department of Defense (monies from ContraFect, TenNor Therapeutics Limited, Adaptive Phage Therapeutics, and BioFire paid to Mayo Clinic); has served as a consultant to Curetis, Specific Technologies, NextGen Diagnostics, Pathoquest, Selux Diagnositcs, and 1928 Diagnostics; reports support for attending meetings and/or travel from ASM Biofilms and International Society of Antimicrobial Chemotherapy; and roles as Chair of ASM Governance Committee and as a member of the ASM Finance Committee. S. S. serves as a Board member for the Evidence Foundation and as Co-Director of Evidence Foundation and US Grade Network (no payments received for this role), and former Chair of the Clinical Guidelines Committee for the American Gastroenterological Association (no payments received for this); receives honoraria for evidence reviews, methodological support, and teaching from the Evidence Foundation; serves on guideline panels for the American Gastroenterological Association (AGA); and receives research funding from the Department of Veterans Affairs Evidence Synthesis Program (paid to their institution). Y. F.-Y. serves as a Board member for the Evidence Foundation; receives honoraria for evidence reviews, methodological support, and teaching from the Evidence Foundation, the AGA for evidence reviews, and the Institute for Clinical and Economic Review (ICER) for committee meetings; serves as a Director for the Evidence Foundation and for the US GRADE Network; and served on an Independent Appraisal Committee for ICER. R. M. serves as a Board member for the Evidence Foundation and receives honoraria for evidence reviews, methodological support, and teaching from the Evidence Foundation. M. H. M. serves as a Board member for the Evidence Foundation; receives honoraria for evidence reviews, methodological support, and teaching from the Evidence Foundation; receives research funding from the Agency for Healthcare Research and Quality, the Endocrine Society, and the Society for Vascular Surgery; has received research funding from the American Society of Hematology and the WHO; and has served as a guideline methodologist for the WHO. A. B. received an honorarium from the ICER. R. A. M. serves as a Board member for the Evidence Foundation; receives honoraria for evidence reviews, methodological support, and teaching from the Evidence Foundation, and ICER for committee meetings; receives research funding from the NIH, the WHO, the American College of Rheumatology, the American Society of Hematology, and Boehringer Ingelheim; serves as Chair of the Midwest Comparative Effectiveness Public Advisory Council of the ICER; serves on the Methods Committee for Kidney Disease Improving Global Outcomes Work Group; serves on the Clinical Guidelines Committee for the Canadian Society of Nephrology; and previously served on the Clinical Guidelines Committee for the American College of Physicians (ACP). D. J. M. reports grants or contracts from NIH (NIH DP2 award), CDC (Shepherd Contract and Co-Investigator on an Epicenter award), AHRQ (R01 on C. difficile), Veteran's Association Health Service Research and Development; support for conference planning and speaking from SHEA/IDSA; and a role as co-lead task force on diagnostic stewardship (unpaid) for SHEA. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
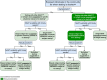
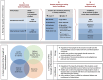
Similar articles
-
The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Antigen Testing.Clin Infect Dis. 2021 Jun 23:ciab557. doi: 10.1093/cid/ciab557. Online ahead of print. Clin Infect Dis. 2021. PMID: 34160592
-
Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2021 Mar 24;3(3):CD013705. doi: 10.1002/14651858.CD013705.pub2. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Jul 22;7:CD013705. doi: 10.1002/14651858.CD013705.pub3. PMID: 33760236 Free PMC article. Updated.
-
The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing (December 2023).Clin Infect Dis. 2024 Jun 27;78(7):e385-e415. doi: 10.1093/cid/ciad646. Clin Infect Dis. 2024. PMID: 38112284 Free PMC article.
-
The Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19: Molecular Diagnostic Testing.Clin Infect Dis. 2021 Jan 22:ciab048. doi: 10.1093/cid/ciab048. Online ahead of print. Clin Infect Dis. 2021. PMID: 33480973 Free PMC article.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
Cited by
-
Clinical impact of rapid molecular diagnostic tests in patients presenting with viral respiratory symptoms: A systematic literature review.PLoS One. 2024 Jun 13;19(6):e0303560. doi: 10.1371/journal.pone.0303560. eCollection 2024. PLoS One. 2024. PMID: 38870136 Free PMC article.
-
Rhabdomyolysis secondary to COVID-19 infection and vaccination: a review of literature.Front Med (Lausanne). 2024 Nov 20;11:1460676. doi: 10.3389/fmed.2024.1460676. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39635585 Free PMC article. Review.
-
Evaluation of Rapid Multiplex Reverse Transcription-Quantitative Polymerase Chain Reaction Assays for SARS-CoV-2 Detection in Individual and Pooled Samples.Life (Basel). 2023 Aug 10;13(8):1717. doi: 10.3390/life13081717. Life (Basel). 2023. PMID: 37629574 Free PMC article.
-
The Impact of Repeating COVID-19 Rapid Antigen Tests on Prevalence Boundary Performance and Missed Diagnoses.Diagnostics (Basel). 2023 Oct 16;13(20):3223. doi: 10.3390/diagnostics13203223. Diagnostics (Basel). 2023. PMID: 37892044 Free PMC article.
-
Diagnostic Accuracy of a SARS-CoV-2 Antigen Test Obtained by Mid-turbinate Nasal Swabs.Cureus. 2025 Jul 1;17(7):e87120. doi: 10.7759/cureus.87120. eCollection 2025 Jul. Cureus. 2025. PMID: 40747206 Free PMC article.
References
-
- US Food and Drug Administration . In vitro diagnostics EUAs—antigen diagnostic tests for SARS-CoV-2. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-em.... Accessed 25 September 2022.
-
- Centers for Disease Control and Prevention . Using antigen tests. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-gu.... Accessed 29 April 2021.
-
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol 2006; 59:1331–2. - PubMed
-
- Centers for Disease Control and Prevention . United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. Available at: https://covid.cdc.gov/covid-data-tracker/#cases_positivity7day. Accessed 25 September 2022.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

