Influenza vaccine effectiveness against influenza A subtypes in Europe: Results from the 2021-2022 I-MOVE primary care multicentre study
- PMID: 36702797
- PMCID: PMC9835407
- DOI: 10.1111/irv.13069
Influenza vaccine effectiveness against influenza A subtypes in Europe: Results from the 2021-2022 I-MOVE primary care multicentre study
Abstract
Background: In 2021-2022, influenza A viruses dominated in Europe. The I-MOVE primary care network conducted a multicentre test-negative study to measure influenza vaccine effectiveness (VE).
Methods: Primary care practitioners collected information on patients presenting with acute respiratory infection. Cases were influenza A(H3N2) or A(H1N1)pdm09 RT-PCR positive, and controls were influenza virus negative. We calculated VE using logistic regression, adjusting for study site, age, sex, onset date, and presence of chronic conditions.
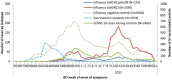
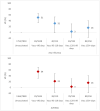
Results: Between week 40 2021 and week 20 2022, we included over 11 000 patients of whom 253 and 1595 were positive for influenza A(H1N1)pdm09 and A(H3N2), respectively. Overall VE against influenza A(H1N1)pdm09 was 75% (95% CI: 43-89) and 81% (95% CI: 45-93) among those aged 15-64 years. Overall VE against influenza A(H3N2) was 29% (95% CI: 12-42) and 25% (95% CI: -41 to 61), 33% (95% CI: 14-49), and 26% (95% CI: -22 to 55) among those aged 0-14, 15-64, and over 65 years, respectively. The A(H3N2) VE among the influenza vaccination target group was 20% (95% CI: -6 to 39). All 53 sequenced A(H1N1)pdm09 viruses belonged to clade 6B.1A.5a.1. Among 410 sequenced influenza A(H3N2) viruses, all but eight belonged to clade 3C.2a1b.2a.2.
Discussion: Despite antigenic mismatch between vaccine and circulating strains for influenza A(H3N2) and A(H1N1)pdm09, 2021-2022 VE estimates against circulating influenza A(H1N1)pdm09 were the highest within the I-MOVE network since the 2009 influenza pandemic. VE against A(H3N2) was lower than A(H1N1)pdm09, but at least one in five individuals vaccinated against influenza were protected against presentation to primary care with laboratory-confirmed influenza.
Keywords: Europe; influenza; influenza vaccine; multicentre study; vaccine effectiveness.
© 2022 The Authors. Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
References
-
- Kissling E, Valenciano M, Buchholz U, et al. Influenza vaccine effectiveness estimates in Europe in a season with three influenza type/subtypes circulating: the I‐MOVE multicentre case‐control study, influenza season 2012/13. Euro Surveill. 2014;19(6):20701. doi: 10.2807/1560-7917.ES2014.19.6.20701 - DOI - PubMed
-
- Kissling E, Valenciano M, Pozo F, et al. 2015/16 I‐MOVE/I‐MOVE+ multicentre case‐control study in Europe: moderate vaccine effectiveness estimates against influenza A(H1N1)pdm09 and low estimates against lineage‐mismatched influenza B among children. Influenza Other Respi Viruses. 2018;12(4):423‐437. doi: 10.1111/irv.12520 - DOI - PMC - PubMed
-
- Valenciano M, Kissling E, Cohen JM, et al. Estimates of pandemic influenza vaccine effectiveness in Europe, 2009–2010: results of Influenza Monitoring Vaccine Effectiveness in Europe (I‐MOVE) multicentre case‐control study. PLoS Med. 2011;8(1):e1000388. doi: 10.1371/journal.pmed.1000388 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

