Inhibition of autophagy and MEK promotes ferroptosis in Lkb1-deficient Kras-driven lung tumors
- PMID: 36702816
- PMCID: PMC9879981
- DOI: 10.1038/s41419-023-05592-8
Inhibition of autophagy and MEK promotes ferroptosis in Lkb1-deficient Kras-driven lung tumors
Abstract
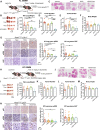
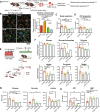
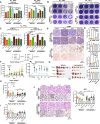
LKB1 and KRAS are the third most frequent co-mutations detected in non-small cell lung cancer (NSCLC) and cause aggressive tumor growth. Unfortunately, treatment with RAS-RAF-MEK-ERK pathway inhibitors has minimal therapeutic efficacy in LKB1-mutant KRAS-driven NSCLC. Autophagy, an intracellular nutrient scavenging pathway, compensates for Lkb1 loss to support Kras-driven lung tumor growth. Here we preclinically evaluate the possibility of autophagy inhibition together with MEK inhibition as a treatment for Kras-driven lung tumors. We found that the combination of the autophagy inhibitor hydroxychloroquine (HCQ) and the MEK inhibitor Trametinib displays synergistic anti-proliferative activity in KrasG12D/+;Lkb1-/- (KL) lung cancer cells, but not in KrasG12D/+;p53-/- (KP) lung cancer cells. In vivo studies using tumor allografts, genetically engineered mouse models (GEMMs) and patient-derived xenografts (PDXs) showed anti-tumor activity of the combination of HCQ and Trametinib on KL but not KP tumors. We further found that the combination treatment significantly reduced mitochondrial membrane potential, basal respiration, and ATP production, while also increasing lipid peroxidation, indicative of ferroptosis, in KL tumor-derived cell lines (TDCLs) and KL tumors compared to treatment with single agents. Moreover, the reduced tumor growth by the combination treatment was rescued by ferroptosis inhibitor. Taken together, we demonstrate that autophagy upregulation in KL tumors causes resistance to Trametinib by inhibiting ferroptosis. Therefore, a combination of autophagy and MEK inhibition could be a novel therapeutic strategy to specifically treat NSCLC bearing co-mutations of LKB1 and KRAS.
© 2023. The Author(s).
Conflict of interest statement
EW is a stockholder in Forma Therapeutics and a founder of Vescor Therapeutics. Other authors have no conflict of interest to declare.
Figures



Similar articles
-
Mutant LKB1 Confers Enhanced Radiosensitization in Combination with Trametinib in KRAS-Mutant Non-Small Cell Lung Cancer.Clin Cancer Res. 2018 Nov 15;24(22):5744-5756. doi: 10.1158/1078-0432.CCR-18-1489. Epub 2018 Aug 1. Clin Cancer Res. 2018. PMID: 30068711
-
Overcoming Resistance to Dual Innate Immune and MEK Inhibition Downstream of KRAS.Cancer Cell. 2018 Sep 10;34(3):439-452.e6. doi: 10.1016/j.ccell.2018.08.009. Cancer Cell. 2018. PMID: 30205046 Free PMC article.
-
Histone Deacetylase 6 Inhibition Exploits Selective Metabolic Vulnerabilities in LKB1 Mutant, KRAS Driven NSCLC.J Thorac Oncol. 2023 Jul;18(7):882-895. doi: 10.1016/j.jtho.2023.03.014. Epub 2023 Mar 22. J Thorac Oncol. 2023. PMID: 36958689 Free PMC article.
-
Targeting KRAS Mutant Non-Small-Cell Lung Cancer: Past, Present and Future.Int J Mol Sci. 2020 Jun 17;21(12):4325. doi: 10.3390/ijms21124325. Int J Mol Sci. 2020. PMID: 32560574 Free PMC article. Review.
-
MEK inhibition in non-small cell lung cancer.Lung Cancer. 2014 Nov;86(2):121-5. doi: 10.1016/j.lungcan.2014.09.005. Epub 2014 Sep 16. Lung Cancer. 2014. PMID: 25257766 Free PMC article. Review.
Cited by
-
New ruthenium-xanthoxylin complex eliminates colorectal cancer stem cells by targeting the heat shock protein 90 chaperone.Cell Death Dis. 2023 Dec 15;14(12):832. doi: 10.1038/s41419-023-06330-w. Cell Death Dis. 2023. PMID: 38102125 Free PMC article.
-
Inhibition of ULK1/2 and KRASG12C controls tumor growth in preclinical models of lung cancer.Elife. 2024 Aug 30;13:RP96992. doi: 10.7554/eLife.96992. Elife. 2024. PMID: 39213022 Free PMC article.
-
Ferroptosis in lung cancer: dual role, multi-level regulation, and new therapeutic strategies.Front Oncol. 2024 Mar 7;14:1360638. doi: 10.3389/fonc.2024.1360638. eCollection 2024. Front Oncol. 2024. PMID: 38515565 Free PMC article. Review.
-
Ferroptosis-related gene HIC1 in the prediction of the prognosis and immunotherapeutic efficacy with immunological activity.Front Immunol. 2023 Jun 14;14:1182030. doi: 10.3389/fimmu.2023.1182030. eCollection 2023. Front Immunol. 2023. PMID: 37388742 Free PMC article.
-
Is Autophagy Targeting a Valid Adjuvant Strategy in Conjunction with Tyrosine Kinase Inhibitors?Cancers (Basel). 2024 Aug 28;16(17):2989. doi: 10.3390/cancers16172989. Cancers (Basel). 2024. PMID: 39272847 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

