Actigraphy-based sleep and activity measurements in intensive care unit patients randomized to ramelteon or placebo for delirium prevention
- PMID: 36702822
- PMCID: PMC9879948
- DOI: 10.1038/s41598-023-28095-0
Actigraphy-based sleep and activity measurements in intensive care unit patients randomized to ramelteon or placebo for delirium prevention
Abstract
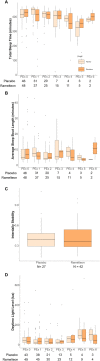
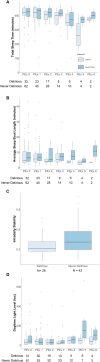
Patients in the ICU often sleep poorly for various reasons, which may predispose to delirium. We previously conducted a clinical trial in which we tested the efficacy of ramelteon, a melatonin-receptor agonist used to treat insomnia, versus placebo, in preventing ICU delirium in patients who underwent elective pulmonary thromboendarterectomy (PTE) surgery. Here we examine sleep, activity, and circadian patterns, measured with actigraphy, to understand changes in these metrics with our intervention and in those with and without delirium. Participants wore wrist actigraphy devices while recovering post-operatively in the ICU. For sleep analysis, we extracted total sleep time and sleep fragmentation metrics over the 22:00 to 06:00 period nightly, and daytime nap duration from the daytime period (0:600 to 22:00) for each participant. For activity analyses, we extracted the following metrics: total daytime activity count (AC), maximum daytime AC, total nighttime AC, and maximum nighttime AC. Next, we performed a nonparametric circadian analysis on ACs over each 24-h day and extracted the following: interdaily stability (IS), intra-daily variability (IV), relative amplitude (RA), and low and high periods of activity (L5 and M10) as well as their start times. These metrics were compared between patients who received ramelteon versus placebo, and between patients who became delirious versus those who did not develop delirium. We additionally made comparisons between groups for daytime and nighttime light levels. No differences in sleep, activity, circadian metrics or light levels were found between drug groups. Delirious patients, when compared to those who were never delirious, had a lower IS (0.35 ± 0.16 vs. 0.47 ± 0.23; P = 0.006). Otherewise, no differences in IV, L5, M10, or RA were found between groups. L5 and M10 activity values increased significantly over the post-extubation for the whole cohort. No differences were found for daytime or nighttime light levels between groups. Overall, ramelteon did not impact sleep or circadian metrics in this cohort. Consistent with clinical experience, delirious patients had less inter-daily stability in their rest-activity rhythms. These data suggest that actigraphy might have value for individual assessment of sleep in the ICU, and for determining and detecting the impact of interventions directed at improving sleep and circadian activity rhythms in the ICU.Trial registration: REGISTERED at CLINICALTRIALS.GOV: NCT02691013. Registered on February 24, 2016 by principal investigator, Dr. Robert L. Owens.
© 2023. The Author(s).
Conflict of interest statement
SAI consults for Eisai, Merck, Idorsia, PureTech. Otherwise, the authors declare that they have no competing interests.
Figures
Similar articles
-
The Prognostic Significance of Sleep and Circadian Rhythm for Myocardial Infarction Outcomes: Case-Control Study.J Med Internet Res. 2025 Feb 4;27:e63897. doi: 10.2196/63897. J Med Internet Res. 2025. PMID: 39903495 Free PMC article.
-
Melatonin and Sleep in Preventing Hospitalized Delirium: A Randomized Clinical Trial.Am J Med. 2018 Sep;131(9):1110-1117.e4. doi: 10.1016/j.amjmed.2018.04.009. Epub 2018 May 3. Am J Med. 2018. PMID: 29729237 Free PMC article. Clinical Trial.
-
Diurnal Rhythm Robustness in Individuals With PTSD and Insomnia and The Association With Sleep.J Biol Rhythms. 2021 Apr;36(2):185-195. doi: 10.1177/0748730420984563. Epub 2021 Jan 20. J Biol Rhythms. 2021. PMID: 33472513 Free PMC article.
-
The role of melatonin and melatonin receptor agonist in the prevention of sleep disturbances and delirium in intensive care unit - a clinical review.Sleep Med. 2020 May;69:127-134. doi: 10.1016/j.sleep.2020.01.019. Epub 2020 Jan 27. Sleep Med. 2020. PMID: 32074506 Review.
-
Pharmacotherapies for sleep disturbances in dementia.Cochrane Database Syst Rev. 2020 Nov 15;11(11):CD009178. doi: 10.1002/14651858.CD009178.pub4. Cochrane Database Syst Rev. 2020. PMID: 33189083 Free PMC article.
Cited by
-
Rest-Activity Rhythm Differences in Acute Rehabilitation Between Poststroke Patients and Non-Brain Disease Controls: Comparative Study.J Med Internet Res. 2024 Jul 4;26:e49530. doi: 10.2196/49530. J Med Internet Res. 2024. PMID: 38963936 Free PMC article.
-
Light levels in a modern intensive care unit: Impact of time of year, window directionality, and outdoor light levels.Chronobiol Int. 2025 Mar;42(3):351-359. doi: 10.1080/07420528.2025.2469885. Epub 2025 Feb 24. Chronobiol Int. 2025. PMID: 39994888
-
Delirium detection using wearable sensors and machine learning in patients with intracerebral hemorrhage.Front Neurol. 2023 Jun 9;14:1135472. doi: 10.3389/fneur.2023.1135472. eCollection 2023. Front Neurol. 2023. PMID: 37360342 Free PMC article.
-
Quantitative Impact of Traditional Open Surgery and Minimally Invasive Surgery on Patients' First-Night Sleep Status in the Intensive Care Unit: Prospective Cohort Study.J Med Internet Res. 2024 Nov 22;26:e56777. doi: 10.2196/56777. J Med Internet Res. 2024. PMID: 39576980 Free PMC article.

