Biomolecular condensates formed by designer minimalistic peptides
- PMID: 36702825
- PMCID: PMC9879991
- DOI: 10.1038/s41467-023-36060-8
Biomolecular condensates formed by designer minimalistic peptides
Abstract
Inspired by the role of intracellular liquid-liquid phase separation (LLPS) in formation of membraneless organelles, there is great interest in developing dynamic compartments formed by LLPS of intrinsically disordered proteins (IDPs) or short peptides. However, the molecular mechanisms underlying the formation of biomolecular condensates have not been fully elucidated, rendering on-demand design of synthetic condensates with tailored physico-chemical functionalities a significant challenge. To address this need, here we design a library of LLPS-promoting peptide building blocks composed of various assembly domains. We show that the LLPS propensity, dynamics, and encapsulation efficiency of compartments can be tuned by changes to the peptide composition. Specifically, with the aid of Raman and NMR spectroscopy, we show that interactions between arginine and aromatic amino acids underlie droplet formation, and that both intra- and intermolecular interactions dictate droplet dynamics. The resulting sequence-structure-function correlation could support the future development of compartments for a variety of applications.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


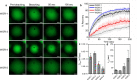
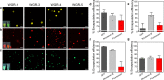
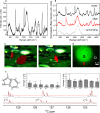
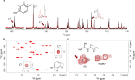
References
-
- Blocher, W. C. & Perry, S. L. Complex coacervate-based materials for biomedicine. Wiley Interdisciplinary Rev.-Nanomed. Nanobiotechnol.9, e1442 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

