In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice
- PMID: 36702898
- PMCID: PMC10264243
- DOI: 10.1038/s41587-022-01558-x
In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice
Abstract
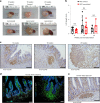
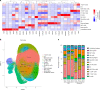
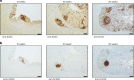
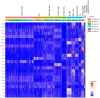
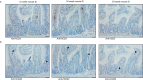
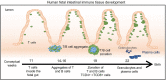
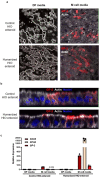
Human intestinal organoids (HIOs) derived from pluripotent stem cells provide a valuable model for investigating human intestinal organogenesis and physiology, but they lack the immune components required to fully recapitulate the complexity of human intestinal biology and diseases. To address this issue and to begin to decipher human intestinal-immune crosstalk during development, we generated HIOs containing immune cells by transplanting HIOs under the kidney capsule of mice with a humanized immune system. We found that human immune cells temporally migrate to the mucosa and form cellular aggregates that resemble human intestinal lymphoid follicles. Moreover, after microbial exposure, epithelial microfold cells are increased in number, leading to immune cell activation determined by the secretion of IgA antibodies in the HIO lumen. This in vivo HIO system with human immune cells provides a framework for future studies on infection- or allergen-driven intestinal diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures








References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

