Study protocol for the sheMATTERS study (iMproving cArdiovascular healTh in new moThERS): a randomized behavioral trial assessing the effect of a self-efficacy enhancing breastfeeding intervention on postpartum blood pressure and breastfeeding continuation in women with hypertensive disorders of pregnancy
- PMID: 36703104
- PMCID: PMC9878496
- DOI: 10.1186/s12884-022-05325-3
Study protocol for the sheMATTERS study (iMproving cArdiovascular healTh in new moThERS): a randomized behavioral trial assessing the effect of a self-efficacy enhancing breastfeeding intervention on postpartum blood pressure and breastfeeding continuation in women with hypertensive disorders of pregnancy
Abstract
Background: Individuals with hypertensive disorders of pregnancy (HDP) have an elevated lifetime risk of chronic hypertension, metabolic syndrome, and premature cardiovascular disease. Because breastfeeding duration and exclusivity have been associated in observational studies with improved cardiovascular health, optimizing breastfeeding in those with HDP might be an unrealized cardio-prevention approach, in particular because individuals with HDP have more breastfeeding challenges. Breastfeeding supportive interventions targeting one's breastfeeding self-efficacy have been shown to improve breastfeeding rates.
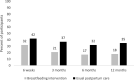
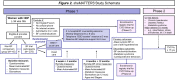
Methods: We designed an open-label, multi-center 1:1 randomized behavioral trial to test whether a previously validated self-efficacy enhancing breastfeeding intervention can improve breastfeeding duration and/or exclusivity, and lower postpartum blood pressure at 12 months. Randomization is computer-generated and stratified by site (four hospitals in Montreal, Quebec and one hospital in Kingston, Ontario; all in Canada). Included are breastfeeding participants with HDP (chronic/gestational hypertension or preeclampsia) who delivered a live singleton infant at > 34 weeks, speak English or French, and have no contraindications to breastfeeding. Informed and written consent is obtained at hospitalization for delivery or a re-admission with hypertension within 1 week of discharge. Participants assigned to the intervention group receive a breastfeeding self-efficacy-based intervention delivered by a trained lactation consultant in hospital, with continued reactive/proactive support by phone or text message for up to 6 months postpartum. Regardless of group assignment, participants are followed for self-reported outcomes, automated office blood pressure, and home blood pressure at several time points with end of follow-up at 12 months.
Discussion: This study will assess whether an intensive nurse-led behavioral intervention can improve breastfeeding rates and, in turn, postpartum blood pressure - an early marker for atherosclerotic cardiovascular disease. If effective, this form of enhanced breastfeeding support, along with closer BP and metabolic surveillance, can be implemented broadly in individuals lactating after HDP.
Trial registration: ClinicalTrials.gov, # NCT04580927 , registered on Oct 9, 2020.
Keywords: Breastfeeding; Hypertensive disorders of pregnancy; Maternal health; Postpartum cardiovascular health; Self-efficacy.
© 2023. The Author(s).
Conflict of interest statement
The authors have no conflict of interests.
Figures

Similar articles
-
Effect of antenatal milk expression education on lactation outcomes in birthing people with pre-pregnancy body mass index ≥25: protocol for a randomized, controlled trial.Int Breastfeed J. 2023 Mar 16;18(1):16. doi: 10.1186/s13006-023-00552-6. Int Breastfeed J. 2023. PMID: 36927811 Free PMC article.
-
Effect on breastfeeding practices of providing in-home lactation support to vulnerable women through the Canada Prenatal Nutrition Program: protocol for a pre/post intervention study.Int Breastfeed J. 2021 Jul 2;16(1):49. doi: 10.1186/s13006-021-00396-y. Int Breastfeed J. 2021. PMID: 34215288 Free PMC article.
-
Blood pressure postpartum (BP2) RCT protocol: Follow-up and lifestyle behaviour change strategies in the first 12 months after hypertensive pregnancy.Pregnancy Hypertens. 2020 Oct;22:1-6. doi: 10.1016/j.preghy.2020.07.001. Epub 2020 Jul 9. Pregnancy Hypertens. 2020. PMID: 32679537
-
Interventions for supporting the initiation and continuation of breastfeeding among women who are overweight or obese.Cochrane Database Syst Rev. 2019 Sep 17;9(9):CD012099. doi: 10.1002/14651858.CD012099.pub2. Cochrane Database Syst Rev. 2019. PMID: 31529625 Free PMC article.
-
Postpartum NSAID Use and Adverse Outcomes among Women with Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-analysis.Am J Perinatol. 2021 Jan;38(1):1-9. doi: 10.1055/s-0040-1713180. Epub 2020 Jul 18. Am J Perinatol. 2021. PMID: 32682329
Cited by
-
Breastfeeding initiation and duration among people with mild chronic hypertension: a secondary analysis of the Chronic Hypertension and Pregnancy trial.Am J Obstet Gynecol MFM. 2023 Sep;5(9):101086. doi: 10.1016/j.ajogmf.2023.101086. Epub 2023 Jul 10. Am J Obstet Gynecol MFM. 2023. PMID: 37437694 Free PMC article. Clinical Trial.
-
Breastfeeding and Maternal and Child Cardiometabolic Outcomes 10-14 Years after Delivery.Breastfeed Med. 2025 Jun;20(6):402-408. doi: 10.1089/bfm.2024.0397. Epub 2025 Mar 11. Breastfeed Med. 2025. PMID: 40067413
-
High-risk pregnancy and risk of breastfeeding failure.J Egypt Public Health Assoc. 2024 Oct 14;99(1):27. doi: 10.1186/s42506-024-00172-w. J Egypt Public Health Assoc. 2024. PMID: 39397190 Free PMC article.
-
Tracking of blood pressure levels from childhood.Pediatr Nephrol. 2025 Feb;40(2):367-376. doi: 10.1007/s00467-024-06485-4. Epub 2024 Aug 28. Pediatr Nephrol. 2025. PMID: 39196350 Review.
-
Breastfeeding patterns among parturients with diabetes: a secondary analysis of the MOMPOD randomized clinical trial.Pregnancy (Hoboken). 2025 Jan;1(1):e12040. doi: 10.1002/pmf2.12040. Epub 2025 Jan 28. Pregnancy (Hoboken). 2025. PMID: 40375954 Free PMC article.
References
-
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ (Clinical research ed) 2007;335(7627):974. doi: 10.1136/bmj.39335.385301.BE. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

