Combined metabolic activators improve cognitive functions in Alzheimer's disease patients: a randomised, double-blinded, placebo-controlled phase-II trial
- PMID: 36703196
- PMCID: PMC9879258
- DOI: 10.1186/s40035-023-00336-2
Combined metabolic activators improve cognitive functions in Alzheimer's disease patients: a randomised, double-blinded, placebo-controlled phase-II trial
Abstract
Background: Alzheimer's disease (AD) is associated with metabolic abnormalities linked to critical elements of neurodegeneration. We recently administered combined metabolic activators (CMA) to the AD rat model and observed that CMA improves the AD-associated histological parameters in the animals. CMA promotes mitochondrial fatty acid uptake from the cytosol, facilitates fatty acid oxidation in the mitochondria, and alleviates oxidative stress.
Methods: Here, we designed a randomised, double-blinded, placebo-controlled phase-II clinical trial and studied the effect of CMA administration on the global metabolism of AD patients. One-dose CMA included 12.35 g L-serine (61.75%), 1 g nicotinamide riboside (5%), 2.55 g N-acetyl-L-cysteine (12.75%), and 3.73 g L-carnitine tartrate (18.65%). AD patients received one dose of CMA or placebo daily during the first 28 days and twice daily between day 28 and day 84. The primary endpoint was the difference in the cognitive function and daily living activity scores between the placebo and the treatment arms. The secondary aim of this study was to evaluate the safety and tolerability of CMA. A comprehensive plasma metabolome and proteome analysis was also performed to evaluate the efficacy of the CMA in AD patients.
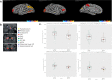
Results: We showed a significant decrease of AD Assessment Scale-cognitive subscale (ADAS-Cog) score on day 84 vs day 0 (P = 0.00001, 29% improvement) in the CMA group. Moreover, there was a significant decline (P = 0.0073) in ADAS-Cog scores (improvement of cognitive functions) in the CMA compared to the placebo group in patients with higher ADAS-Cog scores. Improved cognitive functions in AD patients were supported by the relevant alterations in the hippocampal volumes and cortical thickness based on imaging analysis. Moreover, the plasma levels of proteins and metabolites associated with NAD + and glutathione metabolism were significantly improved after CMA treatment.
Conclusion: Our results indicate that treatment of AD patients with CMA can lead to enhanced cognitive functions and improved clinical parameters associated with phenomics, metabolomics, proteomics and imaging analysis. Trial registration ClinicalTrials.gov NCT04044131 Registered 17 July 2019, https://clinicaltrials.gov/ct2/show/NCT04044131.
Keywords: Alzheimer’s disease; Combined metabolic activators; Multi-omics; Systems biology; Systems medicine.
© 2023. The Author(s).
Conflict of interest statement
AM, JB and MU are the founder and shareholders of ScandiBio Therapeutics. The other authors declare no competing interests.
Figures




References
-
- Trujillo-Estrada L, Jimenez S, De Castro V, Torres M, Baglietto-Vargas D, Moreno-Gonzalez I, et al. In vivo modification of Abeta plaque toxicity as a novel neuroprotective lithium-mediated therapy for Alzheimer's disease pathology. Acta Neuropathol Commun. 2013;1:73. doi: 10.1186/2051-5960-1-73. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

