Two cyclic electron flows around photosystem I differentially participate in C4 photosynthesis
- PMID: 36703198
- PMCID: PMC10069883
- DOI: 10.1093/plphys/kiad032
Two cyclic electron flows around photosystem I differentially participate in C4 photosynthesis
Abstract
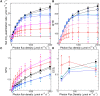
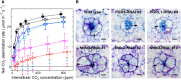
C4 plants assimilate CO2 more efficiently than C3 plants because of their C4 cycle that concentrates CO2. However, the C4 cycle requires additional ATP molecules, which may be supplied by cyclic electron flow (CEF) around photosystem I. One CEF route, which depends on a chloroplast NADH dehydrogenase-like (NDH) complex, is suggested to be crucial for C4 plants despite the low activity in C3 plants. The other route depends on proton gradient regulation 5 (PGR5) and PGR5-like photosynthetic phenotype 1 (PGRL1) and is considered a major CEF route to generate the proton gradient across the thylakoid membrane in C3 plants. However, its contribution to C4 photosynthesis is still unclear. In this study, we investigated the contribution of the two CEF routes to the NADP-malic enzyme subtype of C4 photosynthesis in Flaveria bidentis. We observed that suppressing the NDH-dependent route drastically delayed growth and decreased the CO2 assimilation rate to approximately 30% of the wild-type rate. On the other hand, suppressing the PGR5/PGRL1-dependent route did not affect plant growth and resulted in a CO2 assimilation rate that was approximately 80% of the wild-type rate. Our data indicate that the NDH-dependent CEF substantially contributes to the NADP-malic enzyme subtype of C4 photosynthesis and that the PGR5/PGRL1-dependent route cannot complement the NDH-dependent route in F. bidentis. These findings support the fact that during C4 evolution, photosynthetic electron flow may have been optimized to provide the energy required for C4 photosynthesis.
© American Society of Plant Biologists 2023. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures



Similar articles
-
NDH-Mediated Cyclic Electron Flow Around Photosystem I is Crucial for C4 Photosynthesis.Plant Cell Physiol. 2016 Oct;57(10):2020-2028. doi: 10.1093/pcp/pcw127. Epub 2016 Aug 6. Plant Cell Physiol. 2016. PMID: 27497446
-
Accumulation of the components of cyclic electron flow around photosystem I in C4 plants, with respect to the requirements for ATP.Photosynth Res. 2016 Sep;129(3):261-77. doi: 10.1007/s11120-016-0251-0. Epub 2016 Mar 26. Photosynth Res. 2016. PMID: 27017612 Review.
-
Overproduction of PGR5 enhances the electron sink downstream of photosystem I in a C4 plant, Flaveria bidentis.Plant J. 2020 Jul;103(2):814-823. doi: 10.1111/tpj.14774. Epub 2020 May 6. Plant J. 2020. PMID: 32314445
-
Chloroplast NADH dehydrogenase-like complex-mediated cyclic electron flow is the main electron transport route in C4 bundle sheath cells.New Phytol. 2024 Sep;243(6):2187-2200. doi: 10.1111/nph.19982. Epub 2024 Jul 22. New Phytol. 2024. PMID: 39036838
-
Regulatory network of proton motive force: contribution of cyclic electron transport around photosystem I.Photosynth Res. 2016 Sep;129(3):253-60. doi: 10.1007/s11120-016-0227-0. Epub 2016 Feb 8. Photosynth Res. 2016. PMID: 26858094 Review.
Cited by
-
In vivo two-photon FLIM resolves photosynthetic properties of maize bundle sheath cells.Photosynth Res. 2025 Jan 21;163(1):11. doi: 10.1007/s11120-024-01135-0. Photosynth Res. 2025. PMID: 39836265
-
A pgr5 suppressor screen uncovers two distinct suppression mechanisms and links cytochrome b6f complex stability to PGR5.Plant Cell. 2024 Oct 3;36(10):4245-4266. doi: 10.1093/plcell/koae098. Plant Cell. 2024. PMID: 38781425 Free PMC article.
-
Agrobacterium-mediated transient transformation of Flaveria bidentis leaves: a novel method to examine the evolution of C4 photosynthesis.Plant Methods. 2024 Dec 27;20(1):193. doi: 10.1186/s13007-024-01306-z. Plant Methods. 2024. PMID: 39731143 Free PMC article.
References
-
- Allen JF (2003) Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends Plant Sci 8(1): 15–19 - PubMed
-
- Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25(3): 173–185 - PubMed
-
- Chitty JA, Furbank RT, Marshall JS, Chen Z, Taylor WC (1994) Genetic transformation of the C4 plant, Flaveria bidentis. Plant J 6(6): 949–956
-
- Cruz JA, Avenson TJ, Kanazawa A, Takizawa K, Edwards GE, Kramer DM (2004) Plasticity in light reactions of photosynthesis for energy production and photoprotection. J Exp Bot 56(411): 395–406 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

