The synergistic and enhancive effects of IL-6 and M-CSF to expand and differentiate functional dendritic cells from human monocytes under serum-free condition
- PMID: 36703209
- PMCID: PMC9881386
- DOI: 10.1186/s13036-023-00325-z
The synergistic and enhancive effects of IL-6 and M-CSF to expand and differentiate functional dendritic cells from human monocytes under serum-free condition
Abstract
Background: Dendritic cells (DCs) are differentiated from monocytes, and have a strong ability to perform phagocytosis, present antigens and activate T cell immune response. Therefore, DCs are one of the key factors in fighting cancer in immunotherapy, and it is an important issue to develop a serum-free system for DC differentiation and expansion in vitro for clinical application.
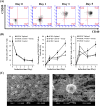
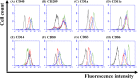
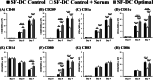
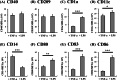
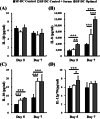
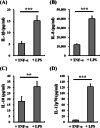
Results: In this study, IL-6 and M-CSF were determined and a concentration combination of cytokines was optimized to develop an optimal DC serum-free differentiation medium (SF-DC Optimal) that can effectively differentiate CD14+ monocytes into CD40+CD209+ DCs. After differentiation, the morphology, growth kinetics, surface antigen expression, phagocytosis ability, cytokine secretion, mixed lymphocyte reaction and stimulation for maturation of the differentiated DCs were checked and confirmed. Importantly, this research is the first report finding that the addition an extra low concentration of IL-6 and M-CSF exhibited a synergistic effect with GM-CSF and IL-4 to generate higher numbers and more fully functional DCs than the addition of GM-CSF and IL-4 only under serum-free condition.
Conclusion: A large number of functional DCs can be generated by using SF-DC Optimal medium and provide an alternative source of DCs for related basic research and clinical applications.
Keywords: Dendritic cell; IL-6; Immunotherapy; M-CSF; Serum-free.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Large-scale production and directed induction of functional dendritic cells ex vivo from serum-free expanded human hematopoietic stem cells.Cytotherapy. 2019 Jul;21(7):755-768. doi: 10.1016/j.jcyt.2019.04.059. Epub 2019 May 17. Cytotherapy. 2019. PMID: 31105040
-
Cytokines in the generation and maturation of dendritic cells: recent advances.Eur Cytokine Netw. 2002 Apr-Jun;13(2):186-99. Eur Cytokine Netw. 2002. PMID: 12101074 Review.
-
1-alpha,25-Dihydroxyvitamin D3 (1,25(OH)(2)D(3)) hampers the maturation of fully active immature dendritic cells from monocytes.Eur J Endocrinol. 2001 Sep;145(3):351-7. doi: 10.1530/eje.0.1450351. Eur J Endocrinol. 2001. PMID: 11517017
-
Dendritic cells generated from CD34+ progenitor cells with flt3 ligand, c-kit ligand, GM-CSF, IL-4, and TNF-alpha are functional antigen-presenting cells resembling mature monocyte-derived dendritic cells.J Immunother. 2000 Jan;23(1):48-58. doi: 10.1097/00002371-200001000-00007. J Immunother. 2000. PMID: 10687137
-
Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties.J Transl Med. 2009 Dec 18;7:109. doi: 10.1186/1479-5876-7-109. J Transl Med. 2009. PMID: 20021667 Free PMC article.
References
-
- Büchler T, Hajek R, Bourkova L, Kovarova L, Musilova R, Bulikova A, Doubek M, Svobodnik A, Mareschova I, Vanova P, Tuzova E, Vidlakova P, Vorlicek J, Penka M. Generation of antigen-loaded dendritic cells in a serum-free medium using different cytokine combinations. Vaccine. 2003;21:877–882. doi: 10.1016/S0264-410X(02)00535-2. - DOI - PubMed
-
- Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, Lu Q, Gao H, Jiang H, Wang H, Yuan D, Ma H, Wang H, Li Z, Zhai B. Chimeric antigen receptor-glypican-3 T-cell therapy for advanced hepatocellular carcinoma: results of phase I trials. Clin Cancer Res. 2020;26:3979–3989. doi: 10.1158/1078-0432.CCR-19-3259. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

