Organophosphorus zwitterions engaged in a conjugated macrocycle on fullerene
- PMID: 36703330
- PMCID: PMC9814461
- DOI: 10.1038/s42004-020-00340-x
Organophosphorus zwitterions engaged in a conjugated macrocycle on fullerene
Abstract
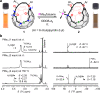
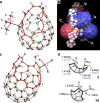
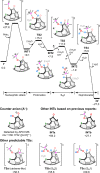
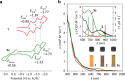
Organophosphorus zwitterions are one of the most important but elusive intermediates for carbon-carbon bond formation in synthetic chemistry and biology. However, a lack of isolated examples due to their lability has hampered in-depth understanding of structures and their reaction mechanisms. In this study, we crystallographically reveal the solid-state structure of a phosha-Michael adduct engaged in a cage-opened C60 skeleton, which is formed as a kinetic product. This compound exhibits dark brown colour in solution with an intense absorption band that extends to 1000 nm, reflecting intramolecular charge transfer transitions. From the 1,2-dicarbonyl moiety on the conjugated orifice, β-oxo-phosphorus ylide is formed as a thermodynamic product. The reaction mechanism that has long been disputed is examined by experimental and theoretical studies, showing a pathway which includes an SN2 reaction as a key step instead of the hitherto considered carbene pathway.
© 2020. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Wittig G, Geisslerg G. Course of reactions of pentaphenylphosphorus and certain derivatives. Justus Liebigs Ann. Chem. 1953;580:44–57. doi: 10.1002/jlac.19535800107. - DOI
LinkOut - more resources
Full Text Sources

